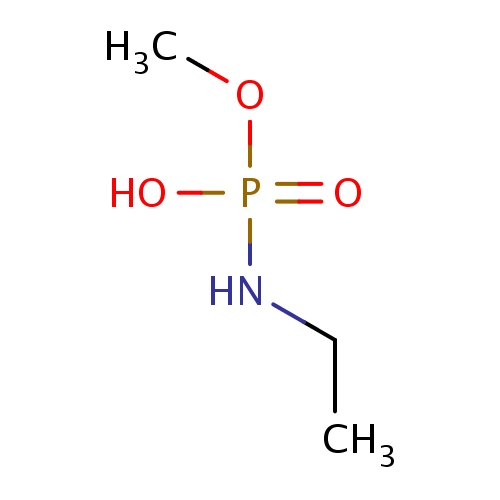
Common name
N-ethyl-methoxy-phosphonamidic acid
IUPAC name
N-ethyl-methoxy-phosphonamidic acid
SMILES
C(C)NP(=O)(OC)O
Common name
N-ethyl-methoxy-phosphonamidic acid
IUPAC name
N-ethyl-methoxy-phosphonamidic acid
SMILES
C(C)NP(=O)(OC)O
INCHI
InChI=1S/C3H10NO3P/c1-3-4-8(5,6)7-2/h3H2,1-2H3,(H2,4,5,6)
FORMULA
C3H10NO3P

Common name
N-ethyl-methoxy-phosphonamidic acid
IUPAC name
N-ethyl-methoxy-phosphonamidic acid
Molecular weight
139.090
clogP
-1.232
clogS
-0.266
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
58.56
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
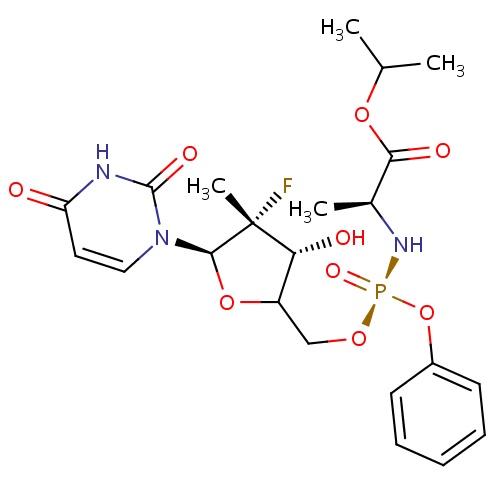
| FDBD01584 | Sofosbuvir |
|
Antiviral Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; | Sofosbuvir is used in combination therapy to treat chronic hepatitis C virus (HCV) infected patients with HCV genoptype 1,2,3, or 4, and to treat HCV and HIV co-infected patients. The combination therapy includes either ribavirin alone or ribavirin and peg-interferon alfa. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2wij_ligand.mol2 | 2wij | 0.6 | -6.13 | CCOP(O)NCC | 9 |
| 5tmn_ligand_3_8.mol2 | 5tmn | 0.533333 | -6.14 | CCN[P@](=O)(O)C | 7 |
| 4tmn_ligand_3_6.mol2 | 4tmn | 0.533333 | -6.11 | CCN[P@@](=O)(C)O | 7 |
| 2wik_ligand.mol2 | 2wik | 0.514286 | -6.34 | CCCNP(O)OCC | 10 |
| 2wig_ligand.mol2 | 2wig | 0.482759 | -5.92 | CCOP(O)NC | 8 |
| 2wik_ligand_3_3.mol2 | 2wik | 0.482759 | -5.63 | C(C)OP(NC)O | 7 |
| 2wij_ligand_1_2.mol2 | 2wij | 0.458333 | -5.71 | P(=O)NCC | 5 |
| 4tmn_ligand_4_33.mol2 | 4tmn | 0.444444 | -6.31 | CCN[P@@](=O)(CC)O | 8 |
| 2wik_ligand_2_0.mol2 | 2wik | 0.392857 | -5.84 | C(CC)NPO | 6 |
204 ,
21

