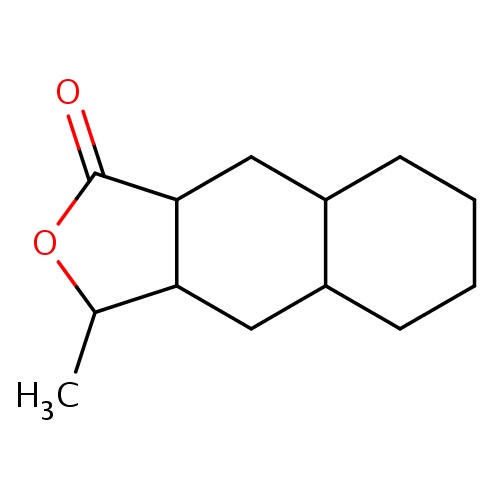
Common name
(3R,3aS,4aS,8aS,9aR)-3-methyl-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-1-one
IUPAC name
(3R,3aS,4aS,8aS,9aR)-3-methyl-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-1-one
SMILES
O1C(C2CC3C(CC2C1=O)CCCC3)C
Common name
(3R,3aS,4aS,8aS,9aR)-3-methyl-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-1-one
IUPAC name
(3R,3aS,4aS,8aS,9aR)-3-methyl-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-1-one
SMILES
O1C(C2CC3C(CC2C1=O)CCCC3)C
INCHI
InChI=1S/C13H20O2/c1-8-11-6-9-4-2-3-5-10(9)7-12(11)13(14)15-8/h8-12H,2-7H2,1H3/t8-,9+,10+,11-,12-/m1/s1
FORMULA
C13H20O2

Common name
(3R,3aS,4aS,8aS,9aR)-3-methyl-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-1-one
IUPAC name
(3R,3aS,4aS,8aS,9aR)-3-methyl-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-1-one
Molecular weight
208.297
clogP
2.469
clogS
-2.077
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
26.3
Number of Rings
3
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
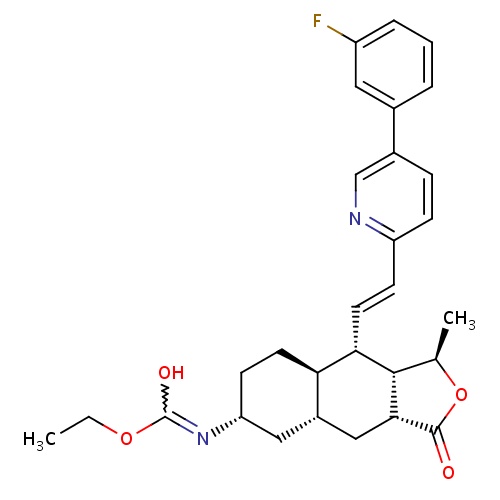
| FDBD01636 | Vorapaxar |
|
Platelet Aggregation Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; | Vorapaxar is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or peripheral arterial disease (PAD). It is usually co-administered with acetylsalicylic acid (ASA) and/or clopidogrel, and should therefore be administered as an addition to these medications as it has not been studied alone. |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2dbl_ligand.mol2 | 2dbl | 0.85 | -9.84 | C1C[C@H](OC(=O)CCC(=O)O)C[C@@H]2CC[C@@H]3[C@@H]([C@@]12C)CC[C@]1([C@H]3CC[C@@H]1C(=O)C)C | 31 |
| 5d1r_ligand.mol2 | 5d1r | 0.828571 | -8.07 | C(CCCCCCCCC)CC(=O)OC(C)C | 18 |
| 4nzn_ligand_1_1.mol2 | 4nzn | 0.771429 | -5.74 | C1(CCCCC1)OC(=O)C | 10 |
| 4hn2_ligand_1_10.mol2 | 4hn2 | 0.771429 | -5.16 | O(C(=O)C)C1CCCCC1 | 10 |
| 1d4y_ligand_5_0.mol2 | 1d4y | 0.761905 | -7.17 | C[C@@H]1[C@H](C[C@@](CC)(OC1=O)CCC)O | 14 |

