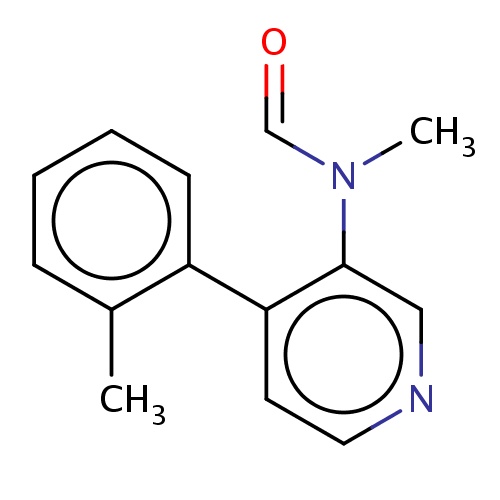
Common name
N-methyl-N-[4-(o-tolyl)-3-pyridyl]formamide
IUPAC name
N-methyl-N-[4-(o-tolyl)-3-pyridyl]formamide
SMILES
O=CN(C)c1c(ccnc1)c2c(cccc2)C
Common name
N-methyl-N-[4-(o-tolyl)-3-pyridyl]formamide
IUPAC name
N-methyl-N-[4-(o-tolyl)-3-pyridyl]formamide
SMILES
O=CN(C)c1c(ccnc1)c2c(cccc2)C
INCHI
InChI=1S/C14H14N2O/c1-11-5-3-4-6-12(11)13-7-8-15-9-14(13)16(2)10-17/h3-10H,1-2H3
FORMULA
C14H14N2O

Common name
N-methyl-N-[4-(o-tolyl)-3-pyridyl]formamide
IUPAC name
N-methyl-N-[4-(o-tolyl)-3-pyridyl]formamide
Molecular weight
226.274
clogP
2.746
clogS
-3.549
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
33.2
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01645 | Netupitant |
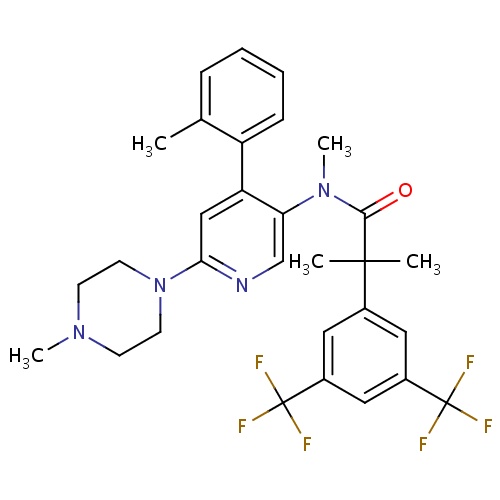
|
Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Netupitant is an antiemitic drug approved by the FDA in October 2014 for use in combination with palonosetron for the prevention of acute and delayed vomiting and nausea associated with cancer chemotherapy including highly emetogenic chemotherapy. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4dvi_ligand_1_1.mol2 | 4dvi | 0.589744 | -7.21 | N(C=O)c1c2nccc(c2ccc1)C | 14 |
| 4n9d_ligand_2_14.mol2 | 4n9d | 0.545455 | -8.27 | C(=O)(Nc1cccnc1)c1ccccc1 | 15 |
| 4n9d_ligand_3_19.mol2 | 4n9d | 0.544444 | -8.54 | Cc1ccc(C(=O)Nc2cccnc2)cc1 | 16 |
| 4n9d_ligand_1_5.mol2 | 4n9d | 0.535211 | -6.52 | C(=O)Nc1cccnc1 | 9 |
| 4n9e_ligand_1_4.mol2 | 4n9e | 0.535211 | -6.44 | C(=O)Nc1cccnc1 | 9 |
| 4n6z_ligand_1_1.mol2 | 4n6z | 0.535211 | -6.30 | c1(cnccc1)NC=O | 9 |
| 5dwr_ligand_1_0.mol2 | 5dwr | 0.535211 | -6.25 | c1(cnccc1)NC=O | 9 |
| 4n70_ligand_1_1.mol2 | 4n70 | 0.535211 | -6.24 | N(C=O)c1cccnc1 | 9 |
| 5ais_ligand_1_4.mol2 | 5ais | 0.535211 | -6.17 | C(=O)Nc1cccnc1 | 9 |
101 ,
11

