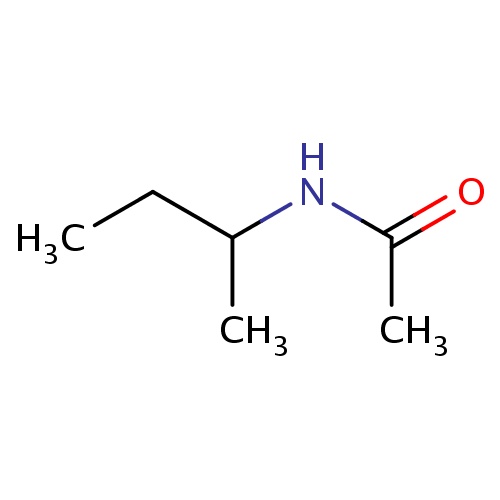
Common name
N-[(1R)-1-methylpropyl]acetamide
IUPAC name
N-[(1R)-1-methylpropyl]acetamide
SMILES
C(C(NC(=O)C)C)C
Common name
N-[(1R)-1-methylpropyl]acetamide
IUPAC name
N-[(1R)-1-methylpropyl]acetamide
SMILES
C(C(NC(=O)C)C)C
INCHI
InChI=1S/C6H13NO/c1-4-5(2)7-6(3)8/h5H,4H2,1-3H3,(H,7,8)/t5-/m1/s1
FORMULA
C6H13NO

Common name
N-[(1R)-1-methylpropyl]acetamide
IUPAC name
N-[(1R)-1-methylpropyl]acetamide
Molecular weight
115.174
clogP
0.588
clogS
-1.440
Frequency
0.0003
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
29.1
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01652 | Cobicistat |
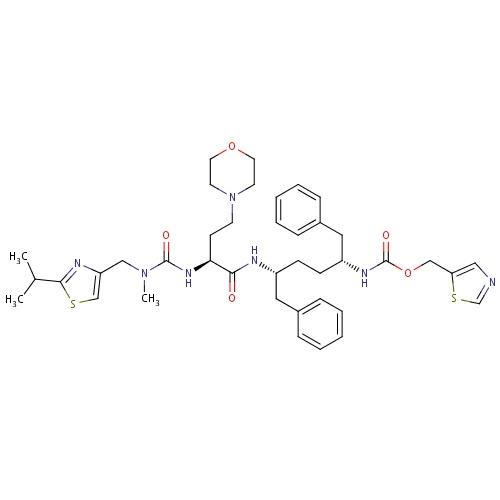
|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2iok_ligand_4_20.mol2 | 2iok | 1 | -6.42 | CC(=O)N[C@H](C)CC | 8 |
| 2iog_ligand_4_120.mol2 | 2iog | 1 | -6.41 | CC[C@@H](C)NC(=O)C | 8 |
| 2woq_ligand_3_0.mol2 | 2woq | 1 | -6.29 | [C@H](C)(NC(=O)C)CC | 8 |
| 3m9f_ligand_4_1750.mol2 | 3m9f | 1 | -6.27 | CC(=O)N[C@@H](C)CC | 8 |
| 1mem_ligand_5_525.mol2 | 1mem | 1 | -5.97 | CC(=O)N[C@H](C)CC | 8 |
412 ,
42

