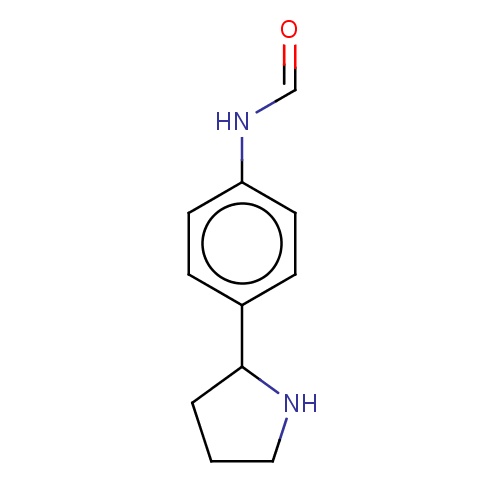
Common name
N-[4-[(2S)-pyrrolidin-2-yl]phenyl]formamide
IUPAC name
N-[4-[(2S)-pyrrolidin-2-yl]phenyl]formamide
SMILES
c1(ccc(cc1)C2NCCC2)NC=O
Common name
N-[4-[(2S)-pyrrolidin-2-yl]phenyl]formamide
IUPAC name
N-[4-[(2S)-pyrrolidin-2-yl]phenyl]formamide
SMILES
c1(ccc(cc1)C2NCCC2)NC=O
INCHI
InChI=1S/C11H14N2O/c14-8-13-10-5-3-9(4-6-10)11-2-1-7-12-11/h3-6,8,11-12H,1-2,7H2,(H,13,14)/t11-/m0/s1
FORMULA
C11H14N2O

Common name
N-[4-[(2S)-pyrrolidin-2-yl]phenyl]formamide
IUPAC name
N-[4-[(2S)-pyrrolidin-2-yl]phenyl]formamide
Molecular weight
190.242
clogP
1.825
clogS
-2.725
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
41.13
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01793 | Ombitasvir |
|
Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; | For use in combination with paritaprevir, ritonavir and dasabuvir for the treatment of HCV genotype 1, and with paritaprevir and ritonavir for the treatment of HCV genotype 4. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2ze1_ligand_2_7.mol2 | 2ze1 | 1 | -6.83 | c1(ccc(cc1)NC=O)[C@@H]1CCC[NH2+]1 | 14 |
| 2ze1_ligand_3_11.mol2 | 2ze1 | 0.970149 | -6.92 | c1(ccc(cc1)NC=O)[C@@H]1CCC[N@@H+]1C | 15 |
| 4zxx_ligand_2_22.mol2 | 4zxx | 0.709302 | -6.29 | c1(cc(ccc1)NC(=O)C)[C@@H]1N(C=O)CCC1 | 17 |
| 2ze1_ligand_1_0.mol2 | 2ze1 | 0.707692 | -6.91 | c1ccc(cc1)[C@@H]1CCC[NH2+]1 | 11 |
| 2qu2_ligand_1_0.mol2 | 2qu2 | 0.707692 | -6.87 | c1cc(ccc1)[C@@H]1CCC[NH2+]1 | 11 |
| 2qu2_ligand_1_1.mol2 | 2qu2 | 0.707692 | -6.78 | c1(ccccc1)[C@H]1CCC[NH2+]1 | 11 |
| 2ze1_ligand_1_1.mol2 | 2ze1 | 0.707692 | -6.65 | c1(ccccc1)[C@@H]1CCC[NH2+]1 | 11 |
| 4zxx_ligand_3_30.mol2 | 4zxx | 0.701149 | -6.52 | CC(=O)N1[C@@H](c2cc(ccc2)NC(=O)C)CCC1 | 18 |
| 2ze1_ligand_2_1.mol2 | 2ze1 | 0.686567 | -6.99 | C[N@H+]1[C@H](c2ccccc2)CCC1 | 12 |
100 ,
11

