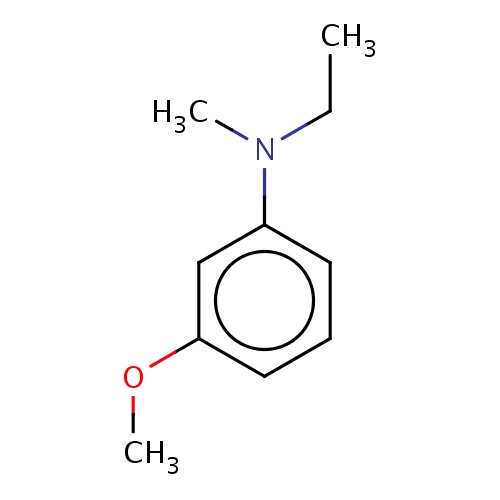
Common name
N-ethyl-3-methoxy-N-methyl-aniline
IUPAC name
N-ethyl-3-methoxy-N-methyl-aniline
SMILES
CCN(c1cccc(c1)OC)C
Common name
N-ethyl-3-methoxy-N-methyl-aniline
IUPAC name
N-ethyl-3-methoxy-N-methyl-aniline
SMILES
CCN(c1cccc(c1)OC)C
INCHI
InChI=1S/C10H15NO/c1-4-11(2)9-6-5-7-10(8-9)12-3/h5-8H,4H2,1-3H3
FORMULA
C10H15NO

Common name
N-ethyl-3-methoxy-N-methyl-aniline
IUPAC name
N-ethyl-3-methoxy-N-methyl-aniline
Molecular weight
165.232
clogP
1.742
clogS
-2.426
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
12.47
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01810 | Osimertinib |
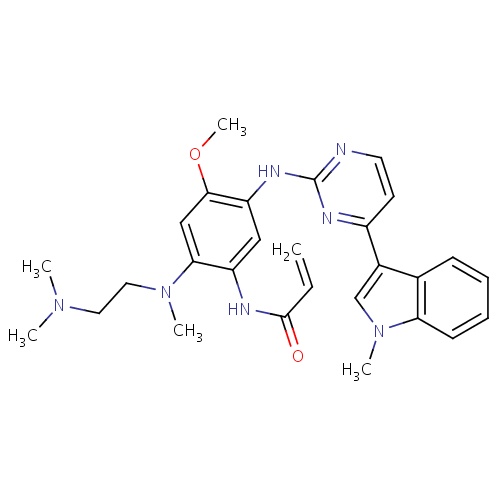
|
Antineoplastic Agents; Protein Kinase Inhibitors; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP3A4 Inhibitors; | Osimertinib is indicated for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA- approved test, who have progressed on or after EGFR-TKI therapy. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1bzf_ligand_3_10.mol2 | 1bzf | 0.846154 | -5.88 | O(C)c1cc(ccc1)NC | 10 |
| 1bzf_ligand_3_12.mol2 | 1bzf | 0.846154 | -5.84 | O(C)c1cccc(c1)NC | 10 |
| 3dek_ligand_4_35.mol2 | 3dek | 0.829787 | -5.68 | CC(=O)Nc1cccc(c1)OC | 12 |
| 4dho_ligand_3_19.mol2 | 4dho | 0.829787 | -5.65 | CC(=O)Nc1cc(OC)ccc1 | 12 |
| 2jko_ligand_2_0.mol2 | 2jko | 0.8125 | -6.44 | O(C)c1cc(N2CC[NH+](CC2)C)ccc1 | 15 |
| 3ekn_ligand_2_0.mol2 | 3ekn | 0.8125 | -6.39 | N1(CC[NH2+]CC1)c1cc(ccc1)OC | 14 |
| 3ika_ligand_2_0.mol2 | 3ika | 0.8125 | -6.31 | C1C[NH+](CCN1c1cc(ccc1)OC)C | 15 |
| 3ekn_ligand_3_5.mol2 | 3ekn | 0.795918 | -6.54 | N1(CC[NH+](CC1)C(C)C)c1cc(OC)ccc1 | 17 |
| 2f1g_ligand_4_209.mol2 | 2f1g | 0.785714 | -5.94 | N(c1ccc(cc1)OC)CC | 11 |
114 ,
12

