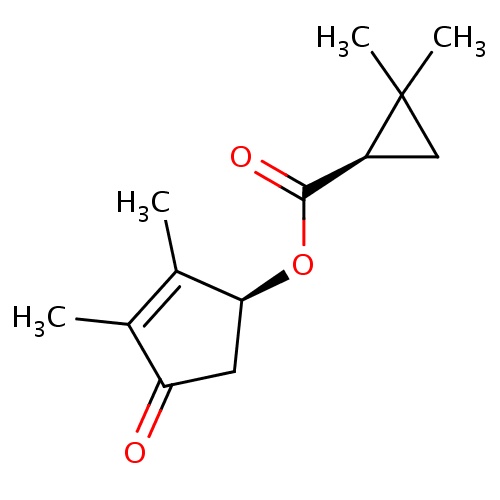
Common name
[(1S)-2,3-dimethyl-4-oxocyclopent-2-en-1-yl] (1R)-2,2-dimethylcyclopropane-1-carboxylate
IUPAC name
[(1S)-2,3-dimethyl-4-oxocyclopent-2-en-1-yl] (1R)-2,2-dimethylcyclopropane-1-carboxylate
SMILES
CC1=C([C@H](CC1=O)OC(=O)[C@H]1C(C1)(C)C)C
Common name
[(1S)-2,3-dimethyl-4-oxocyclopent-2-en-1-yl] (1R)-2,2-dimethylcyclopropane-1-carboxylate
IUPAC name
[(1S)-2,3-dimethyl-4-oxocyclopent-2-en-1-yl] (1R)-2,2-dimethylcyclopropane-1-carboxylate
SMILES
CC1=C([C@H](CC1=O)OC(=O)[C@H]1C(C1)(C)C)C
INCHI
InChI=1S/C13H18O3/c1-7-8(2)11(5-10(7)14)16-12(15)9-6-13(9,3)4/h9,11H,5-6H2,1-4H3/t9-,11-/m0/s1
FORMULA
C13H18O3

Common name
[(1S)-2,3-dimethyl-4-oxocyclopent-2-en-1-yl] (1R)-2,2-dimethylcyclopropane-1-carboxylate
IUPAC name
[(1S)-2,3-dimethyl-4-oxocyclopent-2-en-1-yl] (1R)-2,2-dimethylcyclopropane-1-carboxylate
Molecular weight
222.280
clogP
2.872
clogS
-2.730
Frequency
0.0031
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
43.37
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01854 | cinerins |

|
Insecticide | Insecticide |
| FDBD01855 | cinerin I |

|
Insecticide | Insecticide |
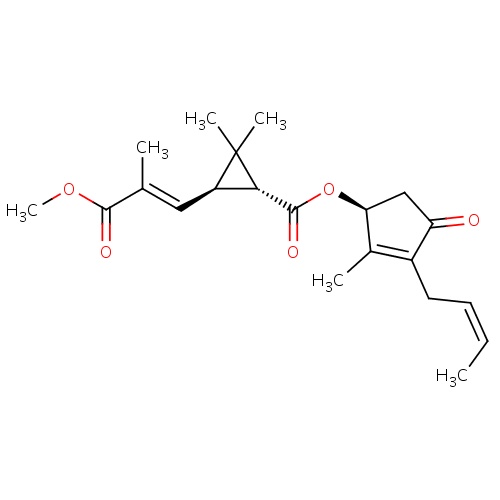
| FDBD01856 | cinerin II |
|
Insecticide | Insecticide |
| FDBD01857 | jasmolin I |

|
Insecticide | Insecticide |
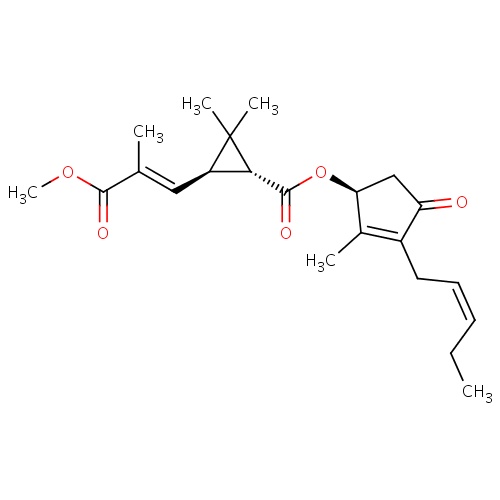
| FDBD01858 | jasmolin II |
|
Insecticide | Insecticide |
| FDBD01859 | pyrethrin I |

|
Insecticide | Insecticide |
| FDBD01860 | pyrethrin II |

|
Insecticide | Insecticide |
| FDBD02263 | chloroprallethrin |

|
Insecticide | Insecticide |
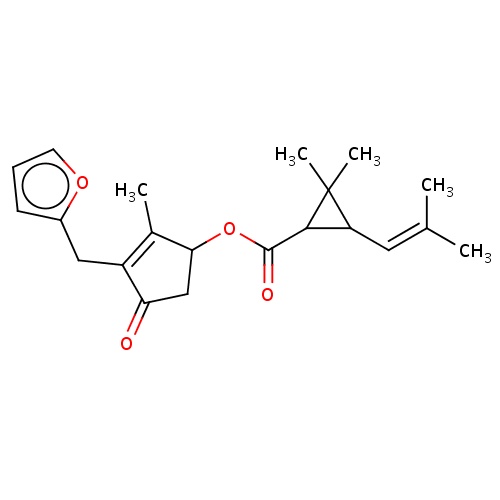
| FDBD02272 | furethrin |
|
Insecticide | Insecticide |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1qca_ligand.mol2 | 1qca | 0.641975 | -10.23 | C1C[C@H]([C@H]([C@@H]2CC[C@]3([C@H]([C@@]12C)[C@@H](C[C@@H]1[C@@]3(C[C@@H](/C/1=C(\CCC=C(C)C)/C(=O)O)OC(=O)C)C)O)C)C)O | 38 |
| 1lnm_ligand.mol2 | 1lnm | 0.575 | -10.46 | O=C1C=C(CO1)[C@H]1CC[C@]2([C@@]1(CC[C@H]1[C@H]2CC[C@H]2[C@@]1(CC[C@@H](C2)O)C)C)O | 28 |
| 1lke_ligand.mol2 | 1lke | 0.567901 | -10.50 | O=C1C=C(CO1)[C@H]1CC[C@]2([C@]1(C)[C@H](O)C[C@H]1[C@H]2CC[C@H]2[C@]1(C)CC[C@@H](C2)O)O | 29 |
| 4j9a_ligand.mol2 | 4j9a | 0.567901 | -9.99 | O=C1C=C(CO1)[C@H]1CC[C@]2([C@]1(C)[C@H](O)C[C@H]1[C@H]2CC[C@H]2[C@]1(C)CC[C@@H](C2)O)O | 29 |
| 4j8t_ligand.mol2 | 4j8t | 0.567901 | -9.86 | O=C1C=C(CO1)[C@H]1CC[C@]2([C@]1(C)[C@H](O)C[C@H]1[C@H]2CC[C@H]2[C@]1(C)CC[C@@H](C2)O)O | 29 |
| 2fxu_ligand_3_460.mol2 | 2fxu | 0.56338 | -6.20 | C[C@H]1[C@H](CC[C@H](CC(=O)/C=C/C)O1)C | 14 |
| 2fxu_ligand_2_81.mol2 | 2fxu | 0.56338 | -5.93 | C([C@H]1CC[C@@H](CO1)C)C(=O)/C=C/C | 13 |
| 3nal_ligand.mol2 | 3nal | 0.561905 | -11.31 | [C@H]12C[C@@H](OC(=O)/C(=C\C)/C)C(=C1[C@@H]1OC(=O)[C@]([C@@]1(O)[C@@H](OC(=O)CCCCCCCCCCC)C[C@@]2(OC(=O)C)C)(O)C)C | 45 |

