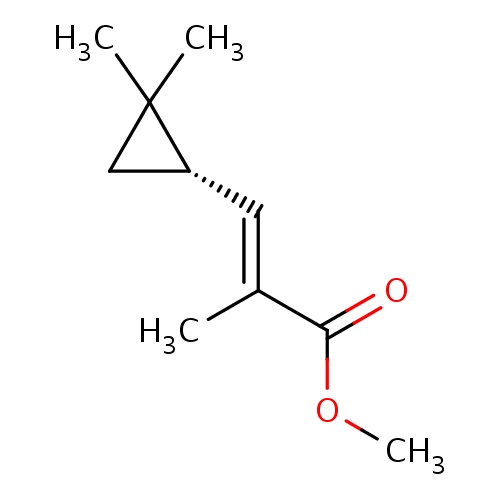
Common name
methyl (E)-3-[(1S)-2,2-dimethylcyclopropyl]-2-methylprop-2-enoate
IUPAC name
methyl (E)-3-[(1S)-2,2-dimethylcyclopropyl]-2-methylprop-2-enoate
SMILES
C1C([C@@H]1/C=C(/C(=O)OC)\C)(C)C
Common name
methyl (E)-3-[(1S)-2,2-dimethylcyclopropyl]-2-methylprop-2-enoate
IUPAC name
methyl (E)-3-[(1S)-2,2-dimethylcyclopropyl]-2-methylprop-2-enoate
SMILES
C1C([C@@H]1/C=C(/C(=O)OC)\C)(C)C
INCHI
InChI=1S/C10H16O2/c1-7(9(11)12-4)5-8-6-10(8,2)3/h5,8H,6H2,1-4H3/b7-5+/t8-/m1/s1
FORMULA
C10H16O2

Common name
methyl (E)-3-[(1S)-2,2-dimethylcyclopropyl]-2-methylprop-2-enoate
IUPAC name
methyl (E)-3-[(1S)-2,2-dimethylcyclopropyl]-2-methylprop-2-enoate
Molecular weight
168.233
clogP
2.184
clogS
-1.789
Frequency
0.0010
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
26.3
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01856 | cinerin II |
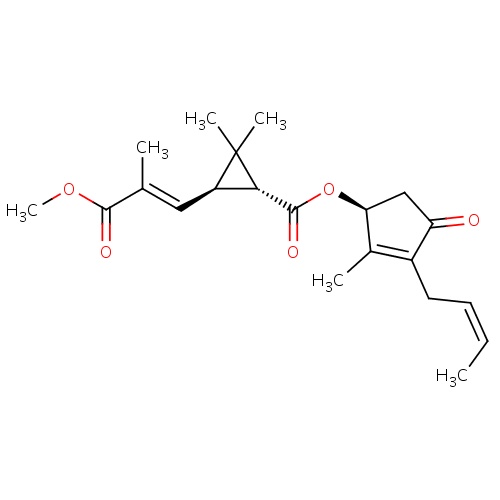
|
Insecticide | Insecticide |
| FDBD01858 | jasmolin II |
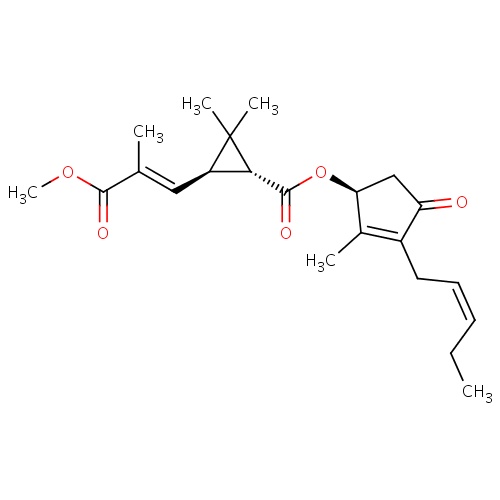
|
Insecticide | Insecticide |
| FDBD01860 | pyrethrin II |

|
Insecticide | Insecticide |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4tjz_ligand.mol2 | 4tjz | 0.666667 | -7.10 | C(=O)(/C=C/CCCCCCC)O | 13 |
| 3cyz_ligand.mol2 | 3cyz | 0.642857 | -7.01 | O=C(C)CCCCC/C=C/C(=O)O | 14 |
| 4tkb_ligand_4_0.mol2 | 4tkb | 0.638889 | -6.34 | CCC/C=C/C(=O)O | 8 |
| 4tkh_ligand_4_0.mol2 | 4tkh | 0.638889 | -6.34 | C(=C\C(=O)O)/CCC | 8 |
| 1hmt_ligand_4_0.mol2 | 1hmt | 0.638889 | -6.28 | C(=O)(O)/C=C/CCC | 8 |
| 4ks5_ligand_2_15.mol2 | 4ks5 | 0.618182 | -5.88 | C1C[C@@H](C=C(C1)C(=O)O)OC | 11 |
| 4ks2_ligand_frag_4.mol2 | 4ks2 | 0.56 | -6.19 | C1CC(=CCC1)C(=O)O | 9 |
| 4ks5_ligand_frag_2.mol2 | 4ks5 | 0.56 | -5.89 | C1CCC=C(C1)C(=O)O | 9 |
| 1lnm_ligand.mol2 | 1lnm | 0.539683 | -10.46 | O=C1C=C(CO1)[C@H]1CC[C@]2([C@@]1(CC[C@H]1[C@H]2CC[C@H]2[C@@]1(CC[C@@H](C2)O)C)C)O | 28 |
100 ,
11

