
Common name
(4S)-2,4-dimethyl-5-phenyl-4H-pyrazol-3-one
IUPAC name
(4S)-2,4-dimethyl-5-phenyl-4H-pyrazol-3-one
SMILES
O=C1[C@@H](C)C(=NN1C)c1ccccc1
Common name
(4S)-2,4-dimethyl-5-phenyl-4H-pyrazol-3-one
IUPAC name
(4S)-2,4-dimethyl-5-phenyl-4H-pyrazol-3-one
SMILES
O=C1[C@@H](C)C(=NN1C)c1ccccc1
INCHI
InChI=1S/C11H12N2O/c1-8-10(12-13(2)11(8)14)9-6-4-3-5-7-9/h3-8H,1-2H3/p+1
FORMULA
C11H12N2O

Common name
(4S)-2,4-dimethyl-5-phenyl-4H-pyrazol-3-one
IUPAC name
(4S)-2,4-dimethyl-5-phenyl-4H-pyrazol-3-one
Molecular weight
188.226
clogP
2.243
clogS
-2.275
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
32.67
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD02986 | pyrametostrobin |
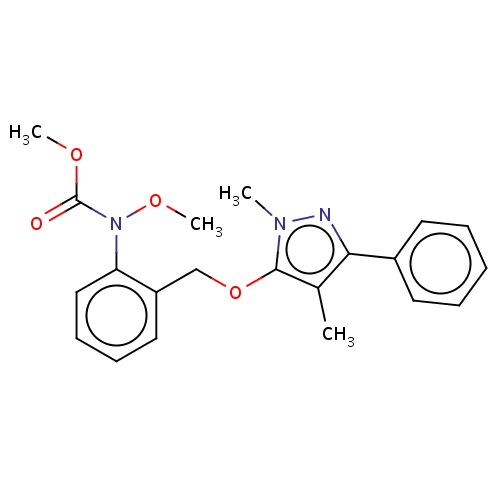
|
Fungicide | Fungicide |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2q2z_ligand_3_15.mol2 | 2q2z | 0.572727 | -7.67 | C(=O)(C)C1=NNC[C@]1(c1ccccc1)C | 15 |
| 2q2z_ligand_2_6.mol2 | 2q2z | 0.572727 | -7.42 | C(=O)(C)C1=NNC[C@H]1c1ccccc1 | 14 |
| 3mlb_ligand_4_49.mol2 | 3mlb | 0.534091 | -7.95 | C(=N\NC(=O)CC)/c1ccccc1 | 13 |
| 1so2_ligand_1_4.mol2 | 1so2 | 0.525862 | -8.39 | C1(=NNC(=O)C[C@H]1C)c1ccccc1 | 14 |
| 1thz_ligand_frag_1.mol2 | 1thz | 0.5 | -5.64 | N1N=C(CC1=O)C | 7 |
| 2g1q_ligand.mol2 | 2g1q | 0.490798 | -9.24 | c1(cc(c(cc1)F)C1=NN([C@@](C1)(c1ccccc1)CCC[NH3+])C(=O)NCC)F | 29 |
| 3gr2_ligand.mol2 | 3gr2 | 0.487603 | -6.46 | O=C1[C@H](CC)C(=NN1c1[nH]nnn1)C | 15 |
| 4kup_ligand.mol2 | 4kup | 0.477612 | -9.29 | C(=N\NC(=O)[C@@H]([NH3+])Cc1c[nH]c2ccccc12)/c1c(C)cc(C)cc1C | 27 |
| 3mlb_ligand_3_23.mol2 | 3mlb | 0.465909 | -7.73 | C(=N\NC(=O)C)/c1ccccc1 | 12 |
138 ,
14

