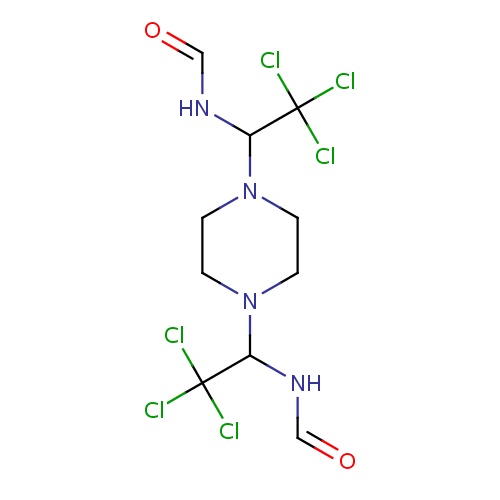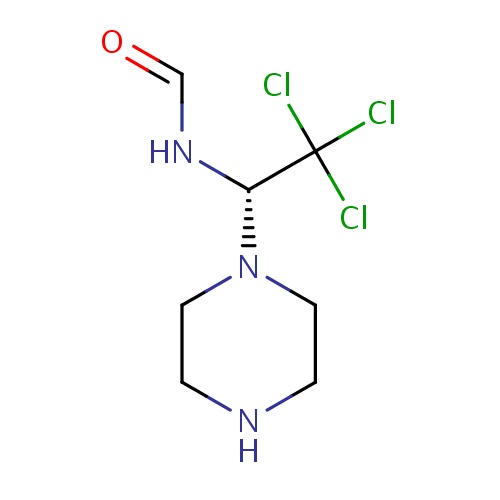
Common name
(1E)-N-[(1R)-2,2,2-trichloro-1-piperazin-1-ylethyl]methanimidic acid
IUPAC name
(1E)-N-[(1R)-2,2,2-trichloro-1-piperazin-1-ylethyl]methanimidic acid
SMILES
C1N(CCNC1)[C@@H](C(Cl)(Cl)Cl)NC=O
Common name
(1E)-N-[(1R)-2,2,2-trichloro-1-piperazin-1-ylethyl]methanimidic acid
IUPAC name
(1E)-N-[(1R)-2,2,2-trichloro-1-piperazin-1-ylethyl]methanimidic acid
SMILES
C1N(CCNC1)[C@@H](C(Cl)(Cl)Cl)NC=O
INCHI
InChI=1S/C7H12Cl3N3O/c8-7(9,10)6(12-5-14)13-3-1-11-2-4-13/h5-6,11H,1-4H2,(H,12,14)/t6-/m0/s1
FORMULA
C7H12Cl3N3O

Common name
(1E)-N-[(1R)-2,2,2-trichloro-1-piperazin-1-ylethyl]methanimidic acid
IUPAC name
(1E)-N-[(1R)-2,2,2-trichloro-1-piperazin-1-ylethyl]methanimidic acid
Molecular weight
260.549
clogP
1.605
clogS
-1.844
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
47.86
Number of Rings
1
Rotatable Bond
3
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2oz2_ligand_frag_0.mol2 | 2oz2 | 0.369565 | -5.48 | C1C[NH+](CCN1C(=O)N)C | 10 |
| 4p6e_ligand_frag_3.mol2 | 4p6e | 0.369565 | -5.33 | C1CN(C(=O)N)CC[NH+]1C | 10 |
| 1mem_ligand_1_0.mol2 | 1mem | 0.346939 | -5.69 | N1(C(=O)NC)CC[NH2+]CC1 | 10 |
| 2oz2_ligand_1_0.mol2 | 2oz2 | 0.346939 | -5.63 | CNC(=O)N1CC[NH+](CC1)C | 11 |