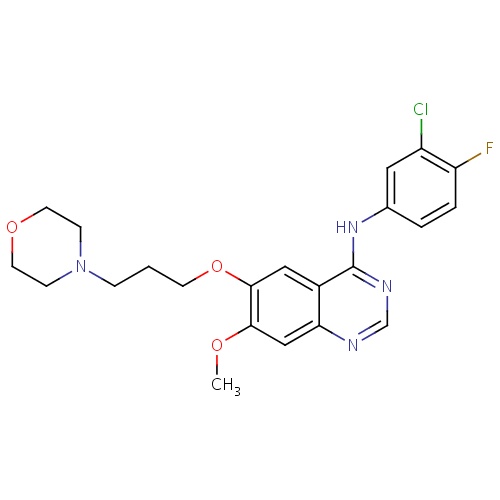
IUPAC name
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine
SMILES
COC1=C(OCCCN2CCOCC2)C=C2C(NC3=CC(Cl)=C(F)C=C3)=NC=NC2=C1
Compound class
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors;
Therapeutic area
For the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of either platinum-based or docetaxel chemotherapies.
Common name
Gefitinib
IUPAC name
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine
SMILES
COC1=C(OCCCN2CCOCC2)C=C2C(NC3=CC(Cl)=C(F)C=C3)=NC=NC2=C1
INCHI
InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27)
FORMULA
C22H24ClFN4O3

Common name
Gefitinib
IUPAC name
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine
Molecular weight
446.902
clogP
4.309
clogS
-7.031
HBond Acceptor
6
HBond Donor
1
Total Polar Surface Area
68.74
Number of Rings
4
Rotatable Bond
8
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00709 | 7-methoxyquinazoline |

|
O(C)c1ccc2c(c1)ncnc2 | 0.0010 |
| FDBF00710 | 4-methylmorpholine |
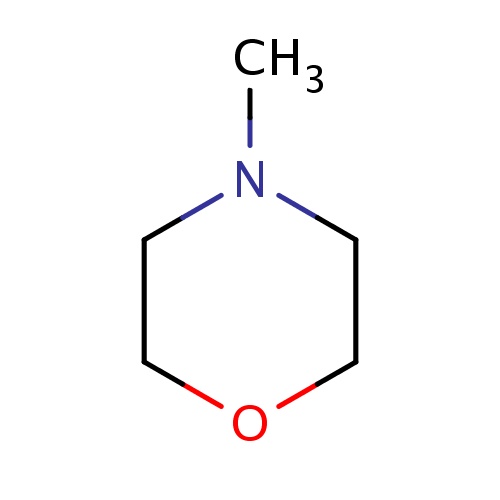
|
O1CCN(CC1)C | 0.0052 |
| FDBF00711 | quinazolin-4-amine |

|
n1c2c(cccc2)c(nc1)N | 0.0031 |
| FDBF00712 | 3-chloro-4-fluoro-aniline |

|
Clc1cc(ccc1F)N | 0.0007 |
| FDBF00713 | 6-methoxyquinazoline |

|
O(C)c1cc2c(ncnc2)cc1 | 0.0010 |
| FDBF00715 | 4-propylmorpholine |
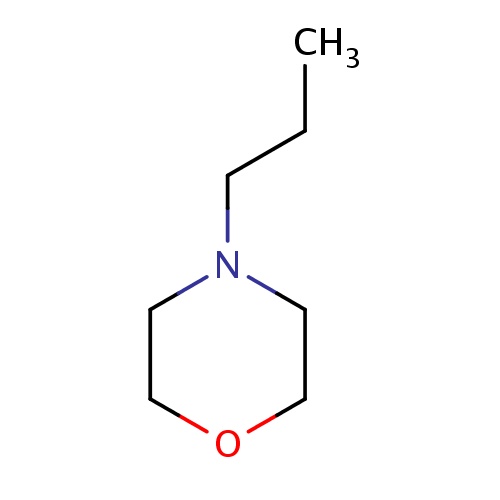
|
O1CCN(CC1)CCC | 0.0010 |
| FDBF00720 | quinazoline |

|
n1c2c(cccc2)cnc1 | 0.0017 |

