
Common name
quinazolin-4-amine
IUPAC name
quinazolin-4-amine
SMILES
n1c2c(cccc2)c(nc1)N
Common name
quinazolin-4-amine
IUPAC name
quinazolin-4-amine
SMILES
n1c2c(cccc2)c(nc1)N
INCHI
InChI=1S/C8H7N3/c9-8-6-3-1-2-4-7(6)10-5-11-8/h1-5H,(H2,9,10,11)
FORMULA
C8H7N3

Common name
quinazolin-4-amine
IUPAC name
quinazolin-4-amine
Molecular weight
145.161
clogP
1.258
clogS
-2.189
Frequency
0.0031
HBond Acceptor
2
HBond Donor
2
Total Polar
Surface Area
51.8
Number of Rings
2
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00203 | Gefitinib |
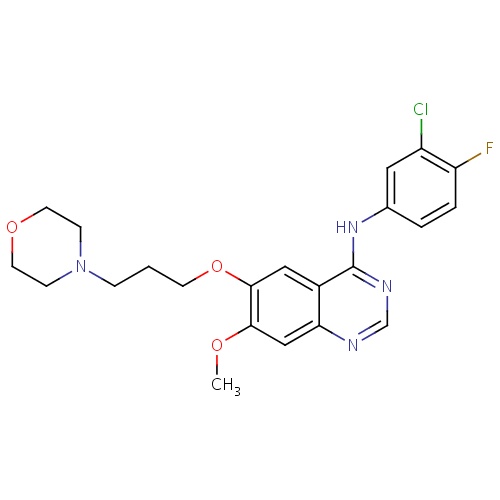
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of either platinum-based or docetaxel chemotherapies. |
| FDBD00230 | Alfuzosin |
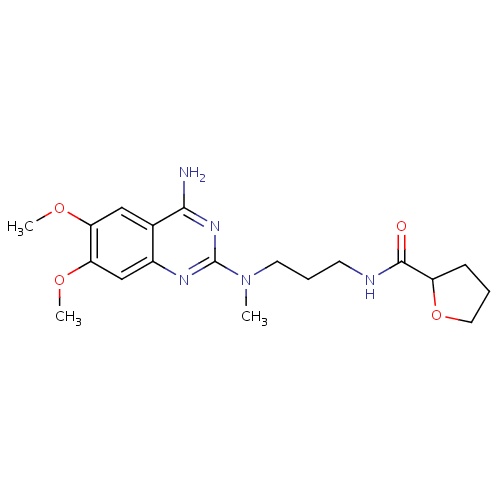
|
Antihypertensive Agents; Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Genito Urinary System and Sex Hormones; Drugs Used in Benign Prostatic Hypertrophy; Urological Agents; CYP3A4 Inhibitors; | For the reduction of urinary obstruction and relief of associated manifestations (eg. sensation of incomplete bladder emptying or straining, urgency, interrupted or weak stream) in patients with symptomatic beningn prostatic hyperplasia. |
| FDBD00332 | Prazosin |
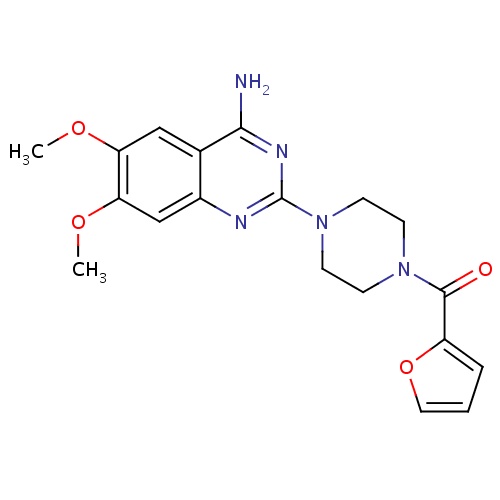
|
Antihypertensive Agents; Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Cardiovascular System; Antiadrenergic Agents, Peripherally Acting; Alpha-Adrenoreceptor Antagonists and Diuretics; | For treatment of hypertension, symptomatic benign prostatic hyperplasia, and severe congestive heart failure. May also be used alone or in combination with β-blockers in the preoperative management of signs and symptoms of pheochromocytoma. |
| FDBD00399 | Erlotinib |
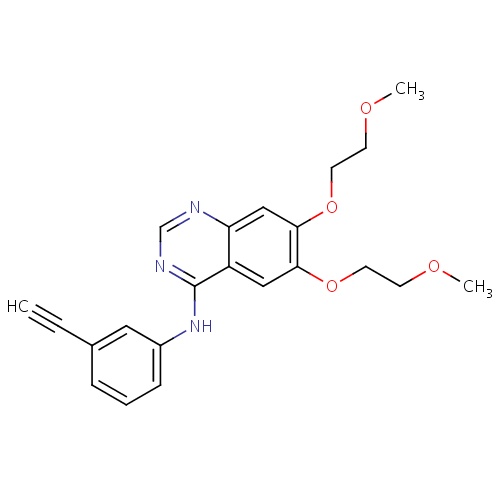
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Also for use, in combination with gemcitabine, as the first-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer. |
| FDBD00455 | Doxazosin |
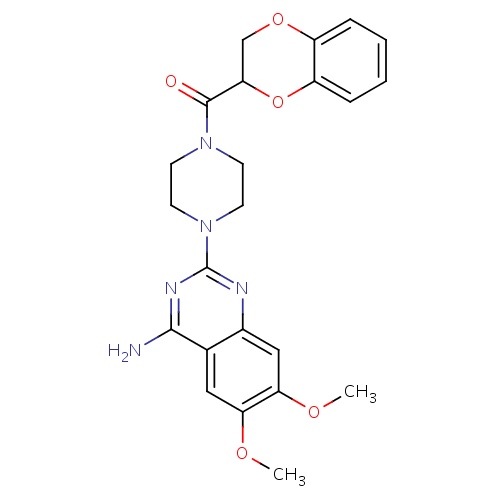
|
Antihypertensive Agents; Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Cardiovascular System; Antiadrenergic Agents, Peripherally Acting; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For treatment and management of mild to moderate hypertension and urinary obstruction symptoms caused by BPH. |
| FDBD01009 | Terazosin |
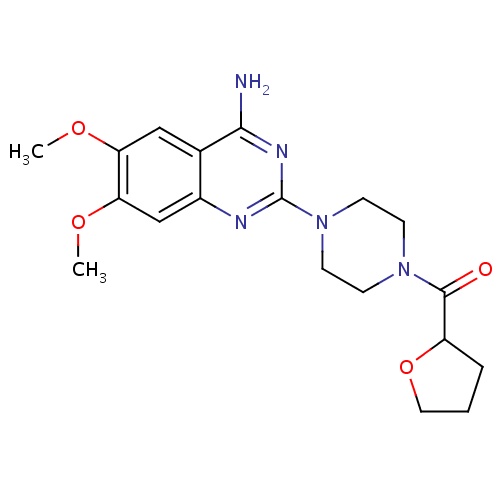
|
Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Genito Urinary System and Sex Hormones; Drugs Used in Benign Prostatic Hypertrophy; Urological Agents; | For the treatment of symptomatic BPH and mild to moderate hypertension. |
| FDBD01101 | Lapatinib |
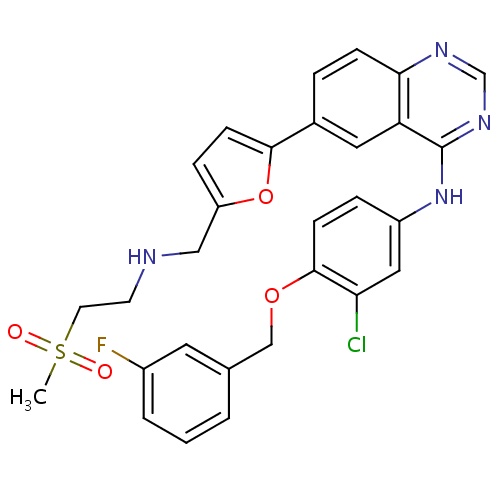
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress the human epidermal receptor type 2 (HER2) protein and who have received prior therapy including an anthracycline, a taxane, and trastuzuma. |
| FDBD01367 | Vandetanib |
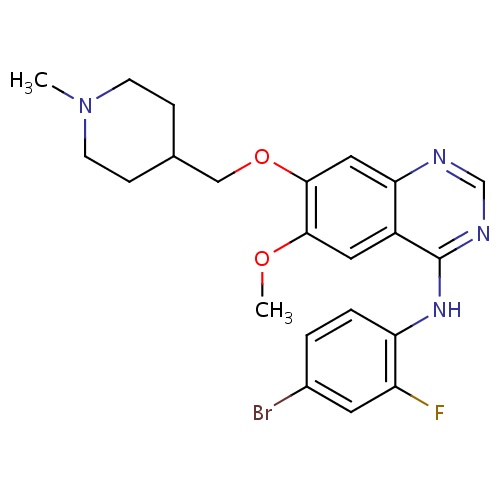
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; CYP3A4 Inhibitors; | Vandetanib is currently approved as an alternative to local therapies for both unresectable and disseminated disease. Because Vandetanib can prolong the Q-T interval, it is contraindicated for use in patients with serious cardiac complications such as congenital long QT syndrome and uncompensated heart failure. |
| FDBD01575 | Afatinib |

|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; | Afatinib is a kinase inhibitor indicated for the first-line treatment of patient with metastatic non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. |
9 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4obq_ligand_frag_0.mol2 | 4obq | 1 | -7.39 | c1cc2c(cc1)c(ncn2)N | 11 |
| 4obo_ligand_frag_1.mol2 | 4obo | 1 | -7.33 | c1ccc2c(c1)c(ncn2)N | 11 |
| 5alr_ligand_1_2.mol2 | 5alr | 1 | -7.31 | c1ccc2c(c1)c(ncn2)N | 11 |
| 4hwo_ligand_frag_4.mol2 | 4hwo | 1 | -7.27 | c1ccc2c(c1)ncnc2N | 11 |
| 4c2v_ligand_1_4.mol2 | 4c2v | 1 | -7.05 | c1(ncnc2ccccc12)N | 11 |
| 2vrx_ligand_1_6.mol2 | 2vrx | 1 | -7.01 | Nc1c2c(cccc2)ncn1 | 11 |
| 2ivu_ligand_1_4.mol2 | 2ivu | 1 | -6.98 | Nc1c2c(cccc2)ncn1 | 11 |
| 1xkk_ligand_1_5.mol2 | 1xkk | 1 | -6.97 | c1ccc2ncnc(c2c1)N | 11 |
| 2qlq_ligand_1_1.mol2 | 2qlq | 1 | -6.97 | c1(c2ccccc2ncn1)N | 11 |
| 2h8h_ligand_1_6.mol2 | 2h8h | 1 | -6.96 | Nc1c2ccccc2ncn1 | 11 |
121 ,
13

