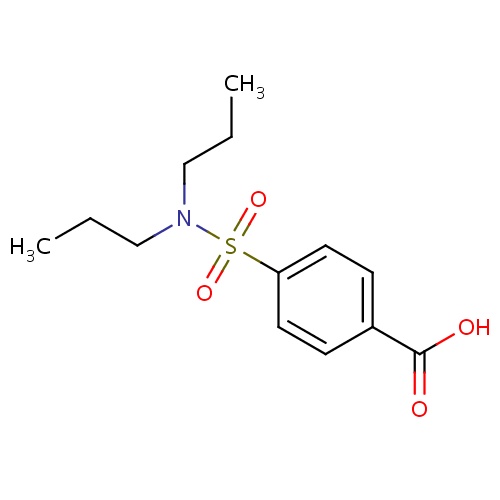
IUPAC name
4-(dipropylsulfamoyl)benzoic acid
SMILES
CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(O)=O
Compound class
Adjuvants, Pharmaceutic; Uricosuric Agents; Musculo-Skeletal System; Antigout Preparations; Preparations Increasing Uric Acid Excretion; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors;
Therapeutic area
For the reduction of serum uric acid concentrations in chronic gouty arthritis and tophaceous gout in patients with frequent disabling gout attacks. Has also been effectively used to promote uric acid excretion in hyperuricemia secondary to the administration of thiazide and related diuretics.
Common name
Probenecid
IUPAC name
4-(dipropylsulfamoyl)benzoic acid
SMILES
CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(O)=O
INCHI
InChI=1S/C13H19NO4S/c1-3-9-14(10-4-2)19(17,18)12-7-5-11(6-8-12)13(15)16/h5-8H,3-4,9-10H2,1-2H3,(H,15,16)
FORMULA
C13H19NO4S

Common name
Probenecid
IUPAC name
4-(dipropylsulfamoyl)benzoic acid
Molecular weight
285.359
clogP
1.196
clogS
-2.816
HBond Acceptor
4
HBond Donor
1
Total Polar
Surface Area
74.68
Number of Rings
1
Rotatable Bond
7
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00007 | propane |

|
C(C)C | 0.2412 |
| FDBF00025 | propan-1-amine |
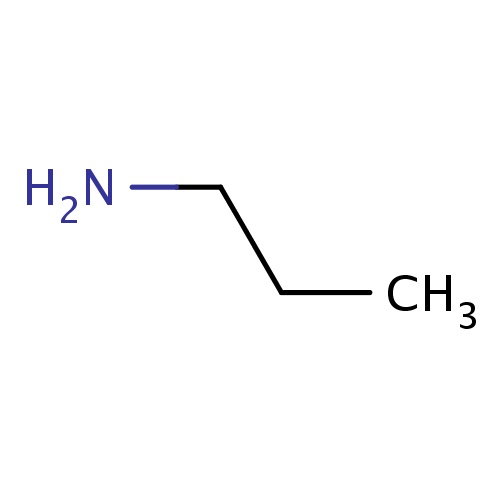
|
CCCN | 0.0292 |
| FDBF00066 | N-methylmethanamine |
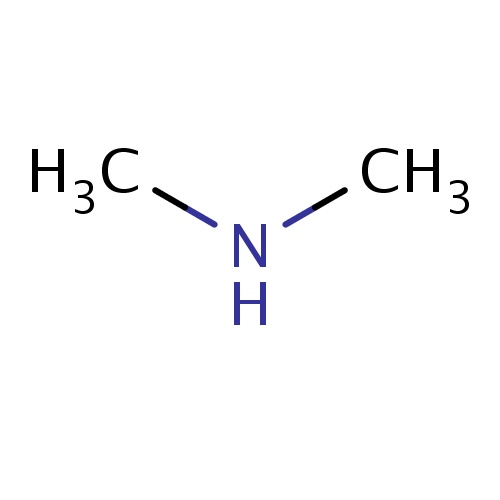
|
N(C)C | 0.0914 |
| FDBF00069 | N-methylpropan-1-amine |
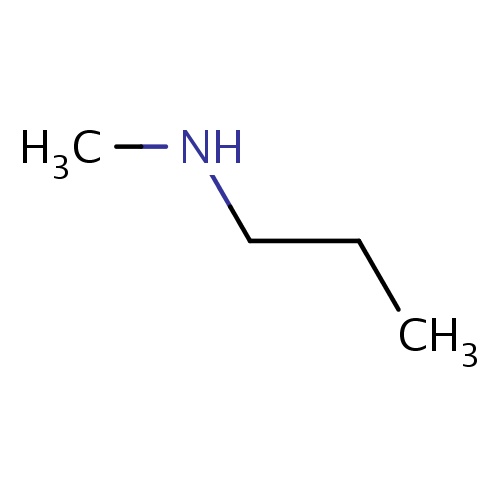
|
N(CCC)C | 0.0148 |
| FDBF00283 | BLAH |
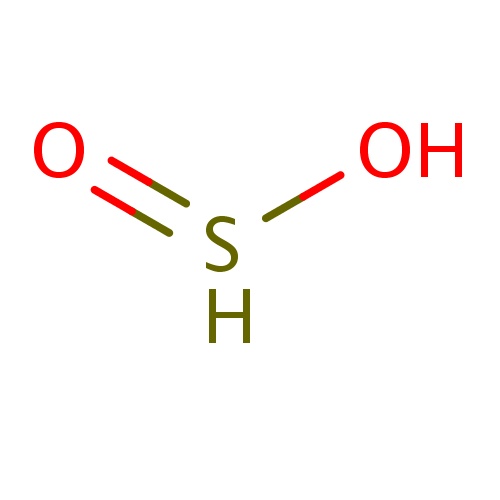
|
S(=O)O | 0.0244 |
| FDBF00576 | N-(dihydroxy-λ3-sulfanyl)methanamine |
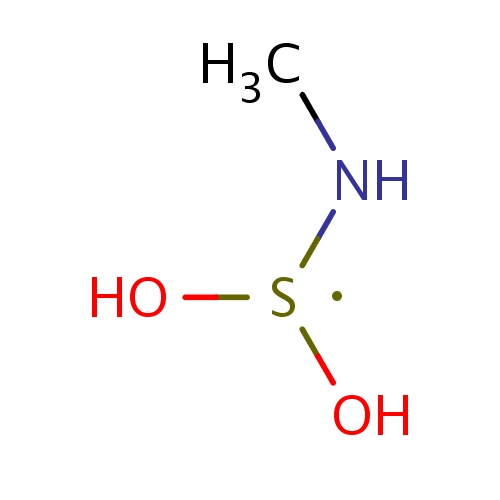
|
CN[S](O)O | 0.0038 |
| FDBF00579 | N-(dihydroxy-λ3-sulfanyl)propan-1-amine |
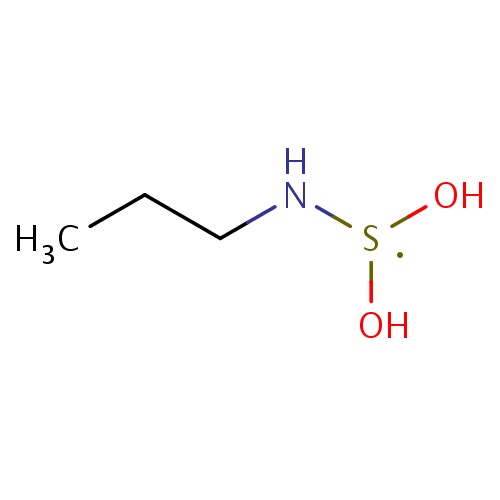
|
CCCN[S](O)O | 0.0010 |
| FDBF00691 | benzoic acid |

|
c1cc(ccc1)C(=O)O | 0.0117 |
| FDBF01627 | N-(dihydroxy-λ3-sulfanyl)-N-methyl-methanamine |
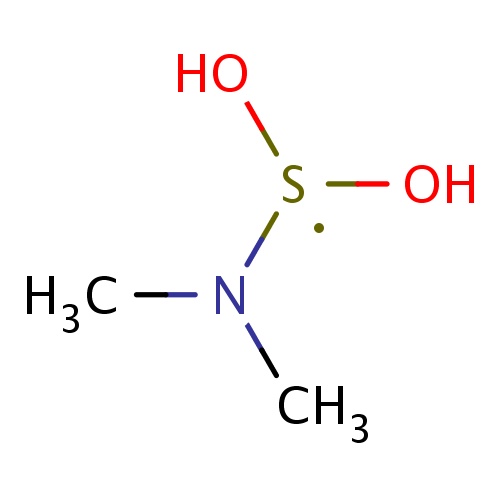
|
[S](O)(O)N(C)C | 0.0041 |
| FDBF02302 | N-(dihydroxy-λ3-sulfanyl)-N-methyl-propan-1-amine |
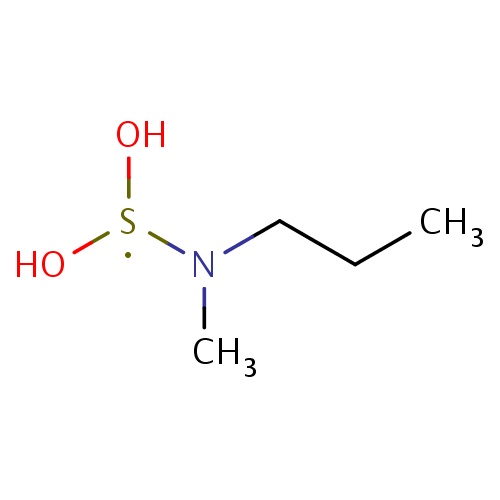
|
C(N(C)[S](O)O)CC | 0.0007 |

