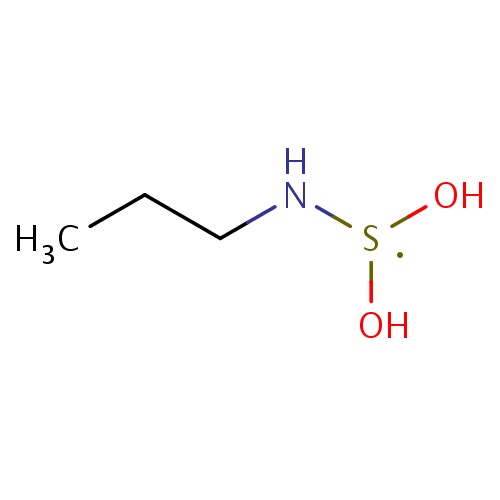
Common name
N-(dihydroxy-λ3-sulfanyl)propan-1-amine
IUPAC name
N-(dihydroxy-λ3-sulfanyl)propan-1-amine
SMILES
CCCN[S](O)O
Common name
N-(dihydroxy-λ3-sulfanyl)propan-1-amine
IUPAC name
N-(dihydroxy-λ3-sulfanyl)propan-1-amine
SMILES
CCCN[S](O)O
INCHI
InChI=1S/C3H10NO2S/c1-2-3-4-7(5)6/h4-6H,2-3H2,1H3
FORMULA
C3H10NO2S

Common name
N-(dihydroxy-λ3-sulfanyl)propan-1-amine
IUPAC name
N-(dihydroxy-λ3-sulfanyl)propan-1-amine
Molecular weight
124.182
clogP
-2.119
clogS
-0.049
Frequency
0.0010
HBond Acceptor
2
HBond Donor
3
Total Polar
Surface Area
52.49
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00165 | Argatroban |
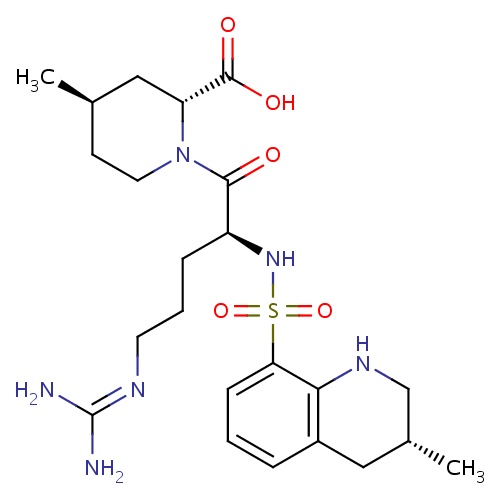
|
Antithrombins; Platelet Aggregation Inhibitors; Direct Thrombin Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; CYP3A4 Inhibitors; | Argatroban is indicated for prevention and treatment of thrombosis caused by heparin induced thrombocytopenia (HIT). It is also indicated for use in patients with, or at risk for, HIT who are undergoing percutaneous coronary intervention. |
| FDBD00885 | Probenecid |
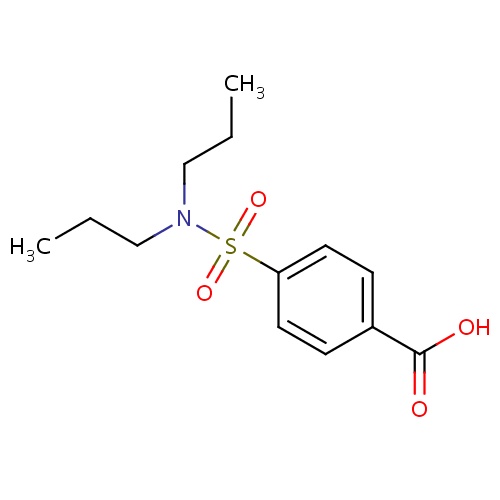
|
Adjuvants, Pharmaceutic; Uricosuric Agents; Musculo-Skeletal System; Antigout Preparations; Preparations Increasing Uric Acid Excretion; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | For the reduction of serum uric acid concentrations in chronic gouty arthritis and tophaceous gout in patients with frequent disabling gout attacks. Has also been effectively used to promote uric acid excretion in hyperuricemia secondary to the administration of thiazide and related diuretics. |
| FDBD01127 | Fosamprenavir |
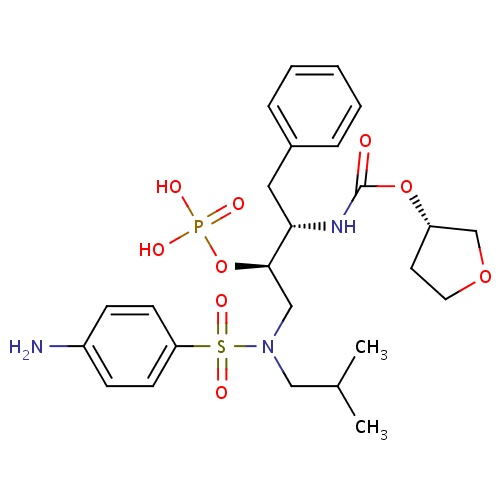
|
Anti-HIV Agents; Protease Inhibitors; HIV Protease Inhibitors; Prodrugs; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection, as well as postexposure prophylaxis of HIV infection in individuals who have had occupational or nonoccupational exposure to potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. The use of fosamprenavir is pending revision due to a potential association between the drug and myocardial infarction and dyslipidemia in HIV infected adults. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4xm6_ligand_2_12.mol2 | 4xm6 | 1 | -6.25 | N([S](O)O)CC(C)C | 8 |
| 4xm8_ligand_2_18.mol2 | 4xm8 | 1 | -6.18 | C(C)(C)CN[S](O)O | 8 |
| 2pj6_ligand_2_24.mol2 | 2pj6 | 1 | -6.05 | [S](O)(O)NCC(C)C | 8 |
| 2pj8_ligand_2_15.mol2 | 2pj8 | 1 | -6.03 | [S](O)(O)NCC(C)C | 8 |
| 2piy_ligand_2_34.mol2 | 2piy | 1 | -6.01 | [S](O)(O)NCC(C)C | 8 |
| 4xm7_ligand_2_17.mol2 | 4xm7 | 1 | -5.98 | C(C)(C)CN[S](O)O | 8 |
| 2pj7_ligand_2_7.mol2 | 2pj7 | 1 | -5.95 | C(C)(C)CN[S](O)O | 8 |
| 2pj9_ligand_2_7.mol2 | 2pj9 | 1 | -5.94 | [S](O)(O)NCC(C)C | 8 |
| 4xm6_ligand_2_5.mol2 | 4xm6 | 1 | -5.91 | C(C)(C)CN[S](O)O | 8 |
158 ,
16

