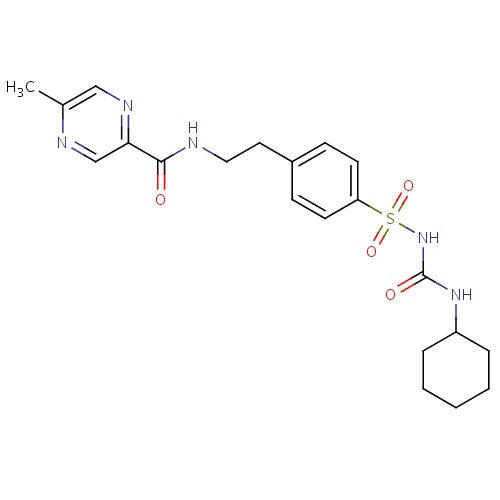
IUPAC name
N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide
SMILES
CC1=CN=C(C=N1)C(=O)NCCC1=CC=C(C=C1)S(=O)(=O)NC(=O)NC1CCCCC1
Compound class
Hypoglycemic Agents; Drugs Used in Diabetes; Alimentary Tract and Metabolism; Blood Glucose Lowering Drugs, Excl. Insulins; Sulfonylureas; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP3A4 Inhibitors;
Therapeutic area
For use as an adjunct to diet for the control of hyperglycemia and its associated symptomatology in patients with non-insulin-dependent diabetes mellitus (NIDDM; type II), formerly known as maturity-onset diabetes, after an adequate trial of dietary therapy has proved unsatisfactory.
Common name
Glipizide
IUPAC name
N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide
SMILES
CC1=CN=C(C=N1)C(=O)NCCC1=CC=C(C=C1)S(=O)(=O)NC(=O)NC1CCCCC1
INCHI
InChI=1S/C21H27N5O4S/c1-15-13-24-19(14-23-15)20(27)22-12-11-16-7-9-18(10-8-16)31(29,30)26-21(28)25-17-5-3-2-4-6-17/h7-10,13-14,17H,2-6,11-12H2,1H3,(H,22,27)(H2,25,26,28)
FORMULA
C21H27N5O4S

Common name
Glipizide
IUPAC name
N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide
Molecular weight
445.535
clogP
1.669
clogS
-5.752
HBond Acceptor
6
HBond Donor
3
Total Polar Surface Area
130.15
Number of Rings
3
Rotatable Bond
7
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
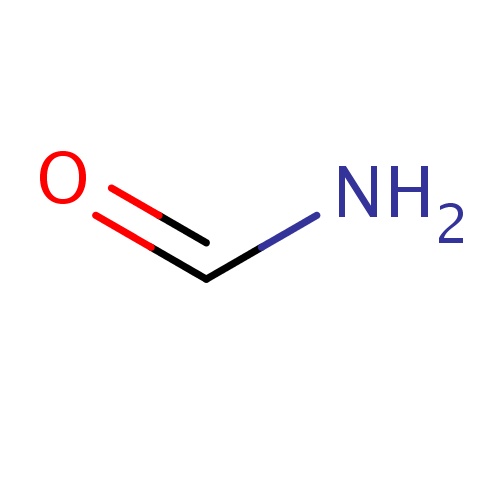
| FDBF00003 | formamide |
|
C(=O)N | 0.1240 |
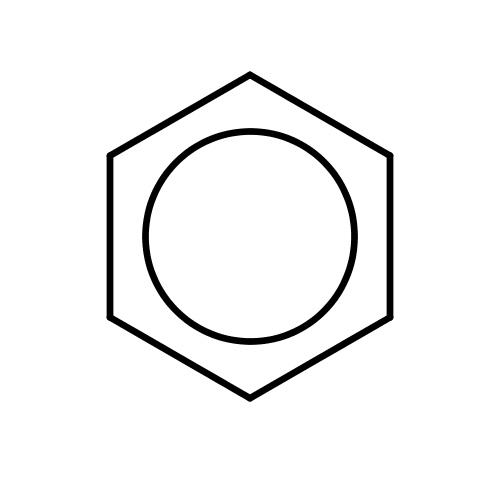
| FDBF00005 | benzene |
|
c1ccccc1 | 0.2824 |
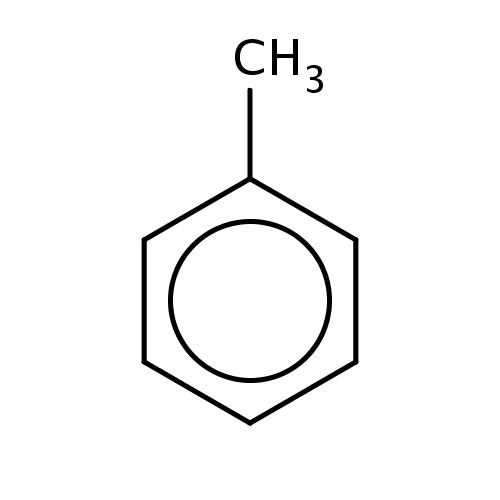
| FDBF00023 | toluene |
|
c1(ccccc1)C | 0.1268 |
| FDBF00141 | ethylbenzene |
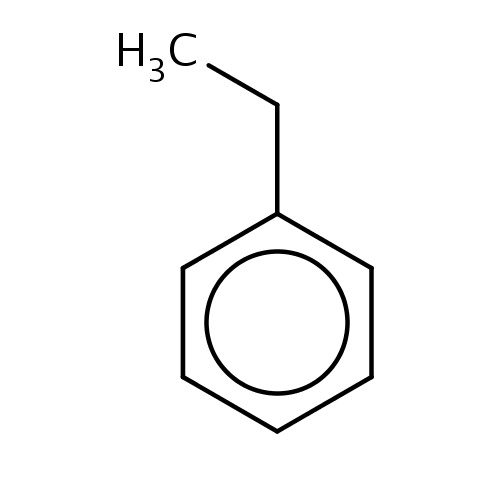
|
c1(ccccc1)CC | 0.0371 |
| FDBF00143 | N-phenethylformamide |

|
c1(ccccc1)CCNC=O | 0.0041 |
| FDBF00355 | cyclohexane |

|
C1CCCCC1 | 0.0127 |
| FDBF02365 | N,5-dimethylpyrazine-2-carboxamide |

|
CNC(=O)c1ncc(nc1)C | 0.0003 |
| FDBF02366 | N-ethyl-5-methyl-pyrazine-2-carboxamide |

|
CCNC(=O)c1ncc(nc1)C | 0.0003 |
| FDBF02367 | 2-methylpyrazine |
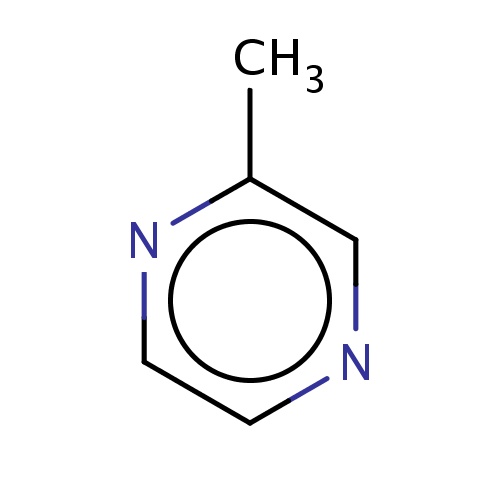
|
n1ccnc(c1)C | 0.0010 |

