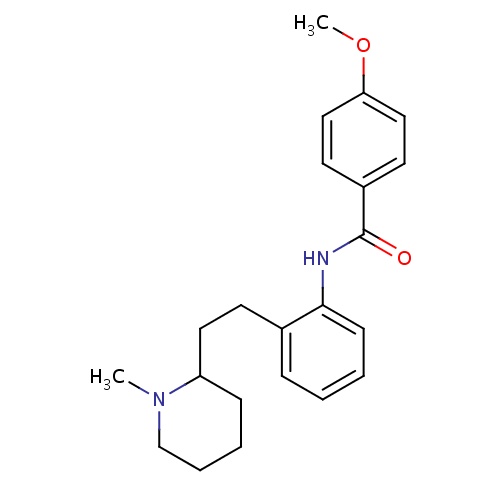
IUPAC name
4-methoxy-N-{2-[2-(1-methylpiperidin-2-yl)ethyl]phenyl}benzamide
SMILES
COC1=CC=C(C=C1)C(=O)NC1=CC=CC=C1CCC1CCCCN1C
Compound class
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ic; CYP2D6 Inducers; CYP2D6 Inducers (strong);
Therapeutic area
Encainide is a class Ic antiarrhythmic agent which was used for management of irregular heartbeats, such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation.
Common name
Encainide
IUPAC name
4-methoxy-N-{2-[2-(1-methylpiperidin-2-yl)ethyl]phenyl}benzamide
SMILES
COC1=CC=C(C=C1)C(=O)NC1=CC=CC=C1CCC1CCCCN1C
INCHI
InChI=1S/C22H28N2O2/c1-24-16-6-5-8-19(24)13-10-17-7-3-4-9-21(17)23-22(25)18-11-14-20(26-2)15-12-18/h3-4,7,9,11-12,14-15,19H,5-6,8,10,13,16H2,1-2H3,(H,23,25)
FORMULA
C22H28N2O2

Common name
Encainide
IUPAC name
4-methoxy-N-{2-[2-(1-methylpiperidin-2-yl)ethyl]phenyl}benzamide
Molecular weight
352.470
clogP
4.095
clogS
-5.481
HBond Acceptor
3
HBond Donor
1
Total Polar Surface Area
41.57
Number of Rings
3
Rotatable Bond
6
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00003 | formamide |
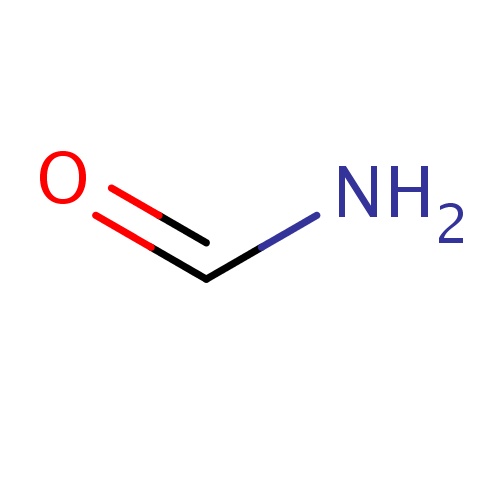
|
C(=O)N | 0.1240 |
| FDBF00005 | benzene |
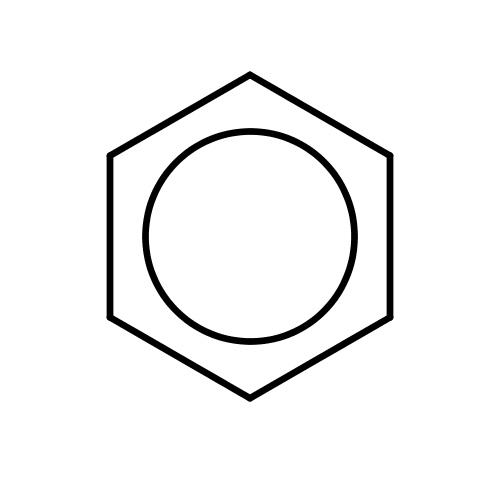
|
c1ccccc1 | 0.2824 |
| FDBF00048 | benzamide |
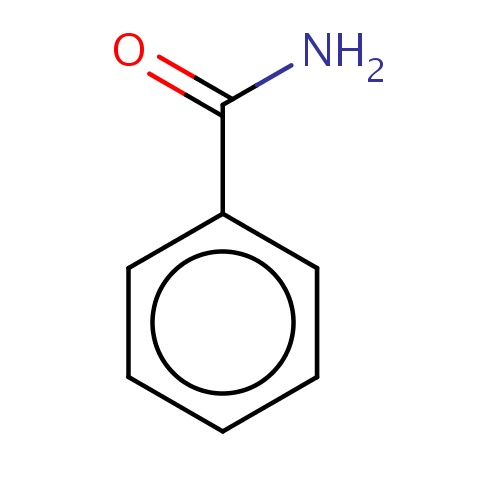
|
O=C(N)c1ccccc1 | 0.0117 |
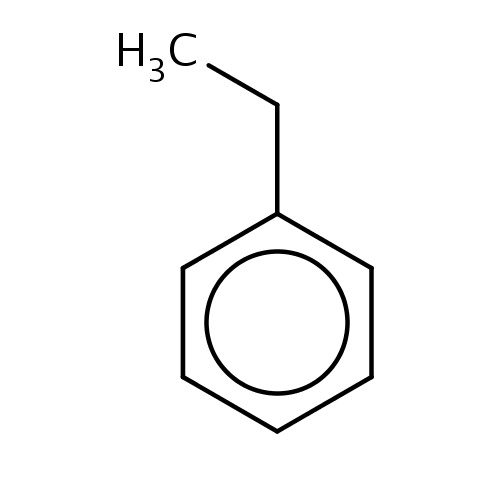
| FDBF00141 | ethylbenzene |
|
c1(ccccc1)CC | 0.0371 |
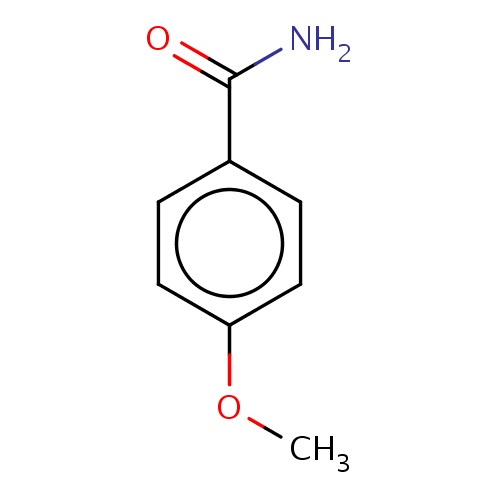
| FDBF01529 | 4-methoxybenzamide |
|
O=C(N)c1ccc(cc1)OC | 0.0014 |
| FDBF01583 | (2R)-1,2-dimethylpiperidine |

|
N1(C(CCCC1)C)C | 0.0010 |
| FDBF01584 | (2R)-2-ethyl-1-methyl-piperidine |

|
N1(C(CCCC1)CC)C | 0.0010 |
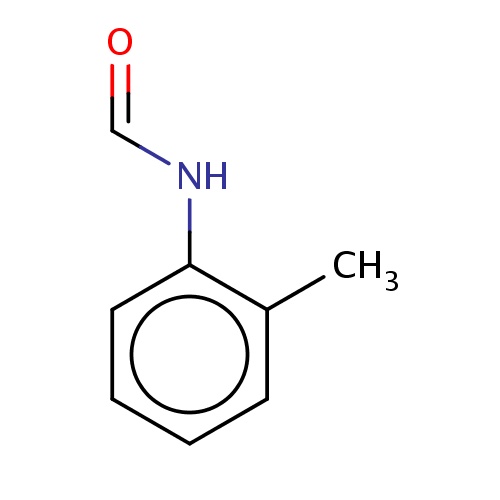
| FDBF01731 | N-(o-tolyl)formamide |
|
O=CNc1c(cccc1)C | 0.0034 |
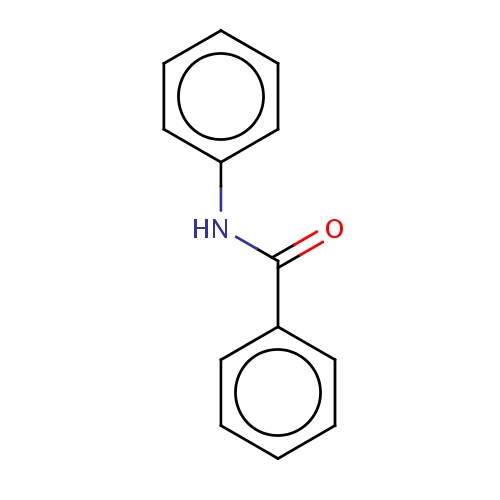
| FDBF01952 | N-phenylbenzamide |
|
c1(ccccc1)C(=O)Nc2ccccc2 | 0.0010 |
| FDBF02772 | 4-methoxy-N-phenyl-benzamide |
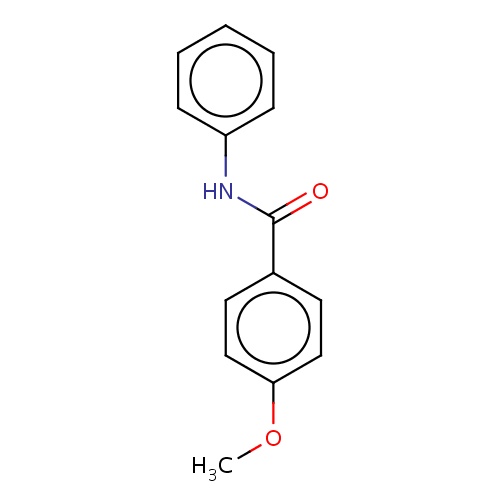
|
c1(ccc(cc1)OC)C(=O)Nc2ccccc2 | 0.0003 |

