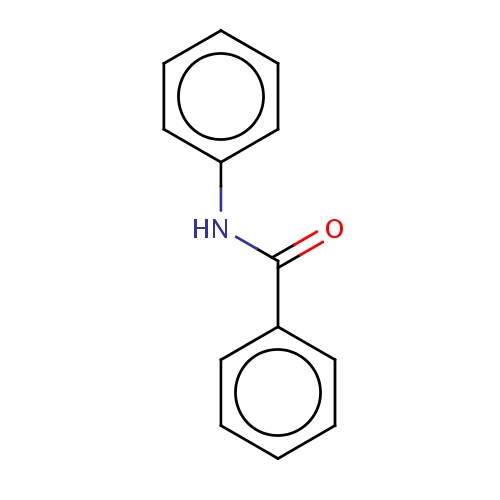
Common name
N-phenylbenzamide
IUPAC name
N-phenylbenzamide
SMILES
c1(ccccc1)C(=O)Nc2ccccc2
Common name
N-phenylbenzamide
IUPAC name
N-phenylbenzamide
SMILES
c1(ccccc1)C(=O)Nc2ccccc2
INCHI
InChI=1S/C13H11NO/c15-13(11-7-3-1-4-8-11)14-12-9-5-2-6-10-12/h1-10H,(H,14,15)
FORMULA
C13H11NO

Common name
N-phenylbenzamide
IUPAC name
N-phenylbenzamide
Molecular weight
197.233
clogP
2.617
clogS
-3.405
Frequency
0.0010
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
29.1
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00732 | Conivaptan |
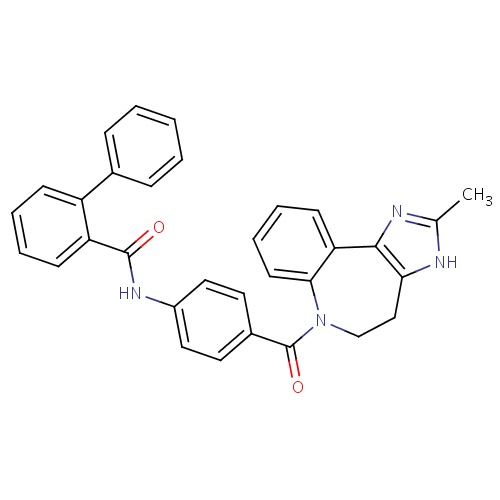
|
Diuretics; Cardiovascular System; Vasopressin Antagonists; CYP3A4 Inhibitors; Antidiuretic Hormone Receptor Antagonists; | For the treatment of euvolemic or hypervolemic hyponatremia (e.g. the syndrome of inappropriate secretion of antidiuretic hormone, or in the setting of hypothyroidism, adrenal insufficiency, pulmonary disorders, etc.) in hospitalized patients. |
| FDBD01073 | Encainide |
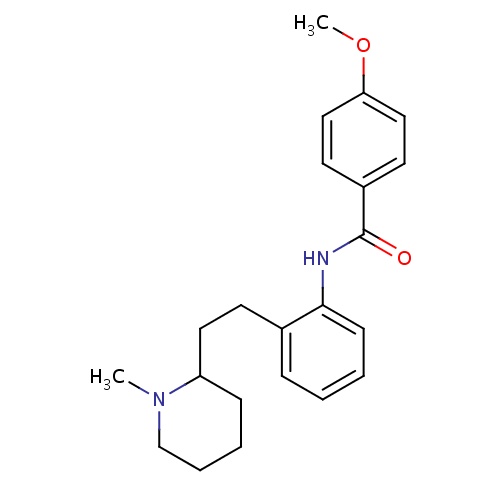
|
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ic; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Encainide is a class Ic antiarrhythmic agent which was used for management of irregular heartbeats, such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation. |
| FDBD02943 | flutolanil |

|
Fungicide | Fungicide |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2hz0_ligand_2_0.mol2 | 2hz0 | 1 | -8.73 | C(=O)(Nc1ccccc1)c1ccccc1 | 15 |
| 5ew3_ligand_2_4.mol2 | 5ew3 | 1 | -8.57 | c1cccc(c1)C(=O)Nc1ccccc1 | 15 |
| 4hct_ligand_2_0.mol2 | 4hct | 1 | -8.41 | C(=O)(Nc1ccccc1)c1ccccc1 | 15 |
| 4hcv_ligand_2_7.mol2 | 4hcv | 1 | -8.25 | C(=O)(Nc1ccccc1)c1ccccc1 | 15 |
| 4i9i_ligand_2_18.mol2 | 4i9i | 1 | -8.25 | c1(ccccc1)NC(=O)c1ccccc1 | 15 |
| 4hcu_ligand_2_7.mol2 | 4hcu | 1 | -8.22 | c1cc(ccc1)NC(=O)c1ccccc1 | 15 |
| 3ii5_ligand_2_13.mol2 | 3ii5 | 1 | -8.21 | O=C(Nc1ccccc1)c1ccccc1 | 15 |
| 4umq_ligand_2_21.mol2 | 4umq | 1 | -8.17 | c1(ccccc1)NC(=O)c1ccccc1 | 15 |
| 4d2t_ligand_2_20.mol2 | 4d2t | 1 | -8.13 | c1(ccccc1)NC(=O)c1ccccc1 | 15 |
| 4jai_ligand_2_5.mol2 | 4jai | 1 | -8.03 | c1ccc(cc1)NC(=O)c1ccccc1 | 15 |
101 ,
11

