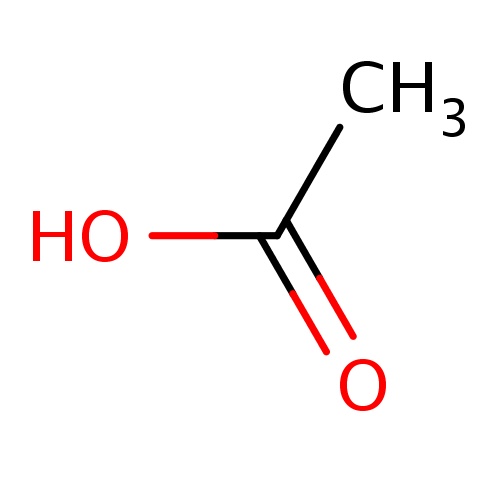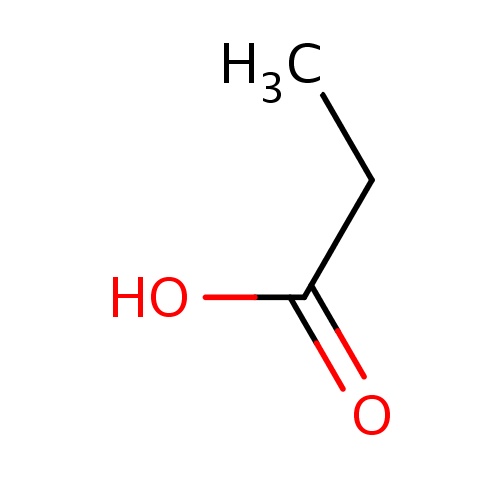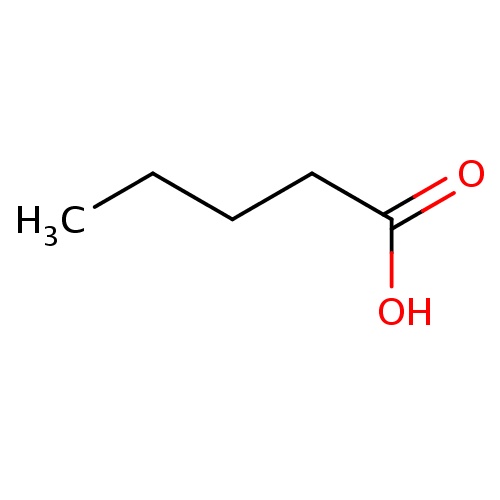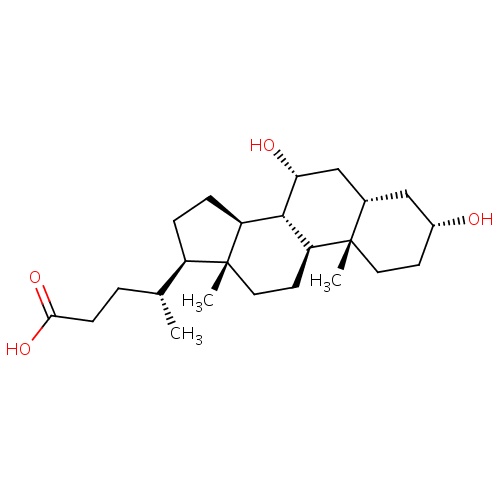
IUPAC name
(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,
SMILES
[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O
Compound class
Gastrointestinal Agents; Cathartics; Alimentary Tract and Metabolism; Bile and Liver Therapy; Bile Acid Preparations; Bile Therapy; CYP3A4 Inhibitors;
Therapeutic area
Chenodiol is indicated for patients with radiolucent stones in well-opacifying gallbladders, in whom selective surgery would be undertaken except for the presence of increased surgical risk due to systemic disease or age. Chenodiol will not dissolve calcified (radiopaque) or radiolucent bile pigment stones.
Common name
Chenodeoxycholic acid
IUPAC name
(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,
SMILES
[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O
INCHI
InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1
FORMULA
C24H40O4

Common name
Chenodeoxycholic acid
IUPAC name
(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,
Molecular weight
392.572
clogP
3.417
clogS
-3.131
HBond Acceptor
4
HBond Donor
3
Total Polar Surface Area
77.76
Number of Rings
4
Rotatable Bond
4