IUPAC name
(3R,4R)-3,6-diamino-4-hydroxy-N-[(3S,6Z,9S,12S,15S)-2,5,8,11,14-pentahydroxy-6-{[(C-hydroxycarbonimidoyl)amino]methylidene}-9,12-bis(hydroxymethyl)-3-[(4R)-2-imino-1,3-diazinan-4-yl]-1,4,7,10,13-pentaazacyclohexadeca-1,4,7,10,13-pentaen-15-yl]hexanimidic acid
SMILES
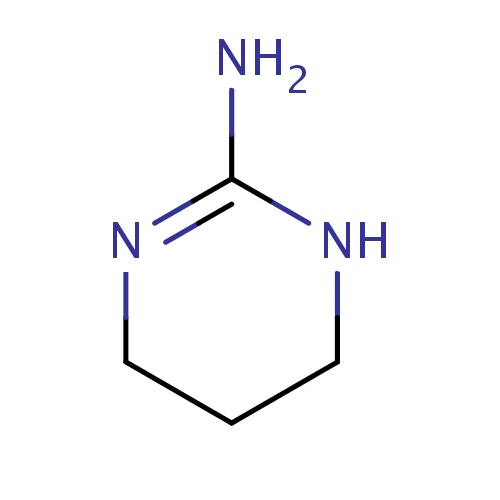
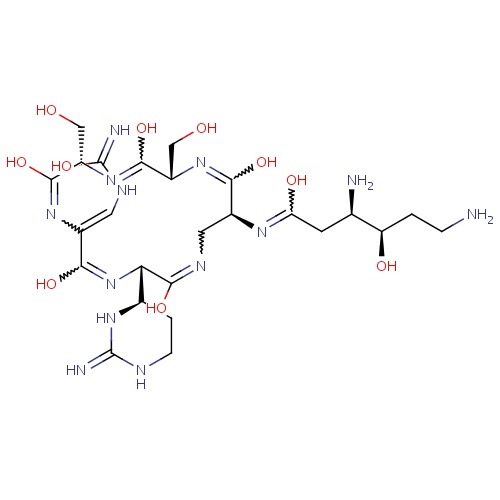
[H]\C(NC(O)=N)=C1\N=C(O)[C@]([H])(CO)N=C(O)[C@]([H])(CO)N=C(O)[C@]([H])(CN=C(O)[C@@]([H])(N=C1O)[C@@]1([H])CCNC(=N)N1)N=C(O)C[C@@]([H])(N)[C@]([H])(O)CCN
Compound class
Anti-Bacterial Agents; Antibiotics, Antitubercular;
Therapeutic area
Common name
Enviomycin
IUPAC name
(3R,4R)-3,6-diamino-4-hydroxy-N-[(3S,6Z,9S,12S,15S)-2,5,8,11,14-pentahydroxy-6-{[(C-hydroxycarbonimidoyl)amino]methylidene}-9,12-bis(hydroxymethyl)-3-[(4R)-2-imino-1,3-diazinan-4-yl]-1,4,7,10,13-pentaazacyclohexadeca-1,4,7,10,13-pentaen-15-yl]hexanimidic acid
SMILES
[H]\C(NC(O)=N)=C1\N=C(O)[C@]([H])(CO)N=C(O)[C@]([H])(CO)N=C(O)[C@]([H])(CN=C(O)[C@@]([H])(N=C1O)[C@@]1([H])CCNC(=N)N1)N=C(O)C[C@@]([H])(N)[C@]([H])(O)CCN
INCHI
InChI=1S/C25H43N13O10/c26-3-1-16(41)10(27)5-17(42)33-12-6-31-23(47)18(11-2-4-30-24(28)37-11)38-20(44)13(7-32-25(29)48)34-21(45)14(8-39)36-22(46)15(9-40)35-19(12)43/h7,10-12,14-16,18,39-41H,1-6,8-9,26-27H2,(H,31,47)(H,33,42)(H,34,45)(H,35,43)(H,36,46)(H,38,44)(H3,28,30,37)(H3,29,32,48)/b13-7-/t10-,11-,12+,14+,15+,16-,18+/m1/s1
FORMULA
C25H43N13O10

Common name
Enviomycin
IUPAC name
(3R,4R)-3,6-diamino-4-hydroxy-N-[(3S,6Z,9S,12S,15S)-2,5,8,11,14-pentahydroxy-6-{[(C-hydroxycarbonimidoyl)amino]methylidene}-9,12-bis(hydroxymethyl)-3-[(4R)-2-imino-1,3-diazinan-4-yl]-1,4,7,10,13-pentaazacyclohexadeca-1,4,7,10,13-pentaen-15-yl]hexanimidic acid
Molecular weight
685.690
clogP
-3.221
clogS
1.297
HBond Acceptor
16
HBond Donor
19
Total Polar Surface Area
412.29
Number of Rings
2
Rotatable Bond
11