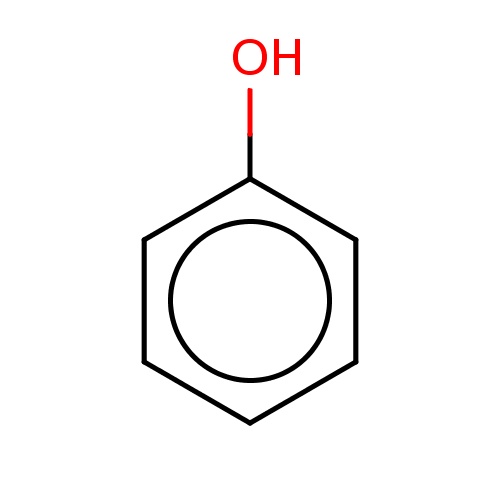
Common name
phenol
IUPAC name
phenol
SMILES
c1ccc(cc1)O
Common name
phenol
IUPAC name
phenol
SMILES
c1ccc(cc1)O
INCHI
InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H
FORMULA
C6H6O

Common name
phenol
IUPAC name
phenol
Molecular weight
94.111
clogP
1.498
clogS
-0.975
Frequency
0.0897
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
20.23
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01349 | Permethrin |

|
Enzyme Inhibitors; Insecticides; Ectoparasiticides for Topical Use, Incl. Insecticides; Ectoparaciticides, Insecticides and Repellents; Antiparasitic Products, Insecticides and Repellents; Ectoparasiticides, Incl. Scabicides; Ectoparasiticides, Incl. Scabicides, Insecticides and Repellents; Pyrethrines, Incl. Synthetic Compounds; Pyrethrins and Pyrethroids; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | For the treatment of infestation with . |
| FDBD01350 | Ospemifene |

|
Estrogen Antagonists; Selective Estrogen Receptor Modulators; Sex Hormones and Modulators of the Genital System; Genito Urinary System and Sex Hormones; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | Ospemifene is used for the treatment of moderate to dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause. |
| FDBD01352 | Iloperidone |
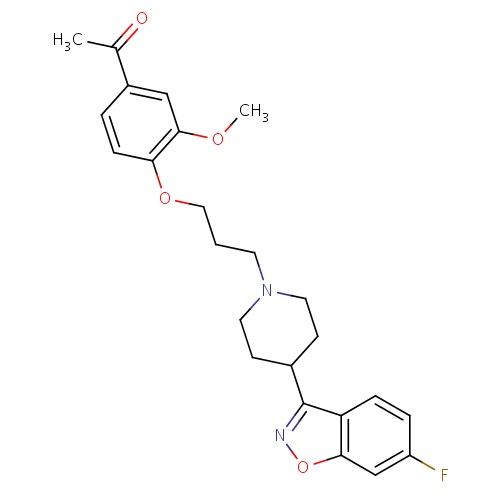
|
Antipsychotic Agents; Adrenergic alpha-1 Receptor Antagonists; Nervous System; Psycholeptics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | Treatment of acute schizophrenia. |
| FDBD01371 | Apremilast |

|
Anti-Inflammatory Agents, Non-Steroidal; Immunosuppressive Agents; Antineoplastic and Immunomodulating Agents; Selective Immunosuppressants; CYP3A4 Inhibitors; | Investigated for use/treatment in psoriasis and psoriatic disorders. |
| FDBD01384 | Tapentadol |
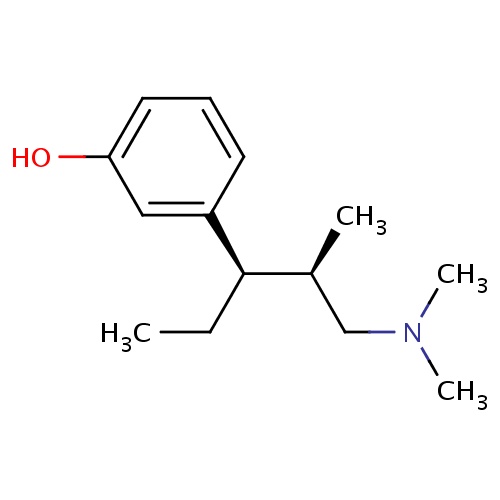
|
Analgesics; Nervous System; Opioids; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | The immediate-release formulation of tapentadol is indicated for the relief of moderate to severe acute pain. The long-acting formulation serves as a continuous, around-the-clock analgesic that is indicated for the relief of moderate to severe chronic pain or neuropathic pain associated with diabetic peripheral neuropathy. |
| FDBD01385 | Silodosin |
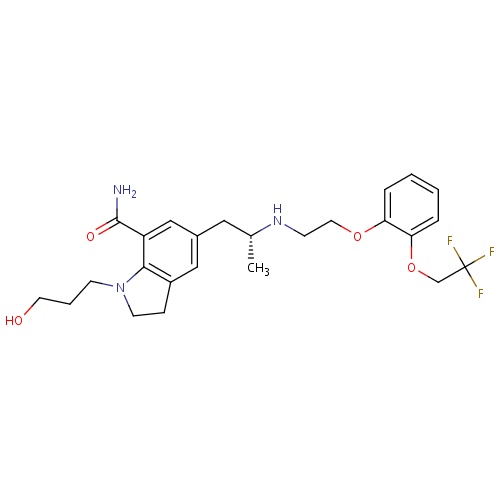
|
Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Genito Urinary System and Sex Hormones; Drugs Used in Benign Prostatic Hypertrophy; Urological Agents; CYP3A4 Inhibitors; | Treatment for symptomatic relief of benign prostatic hyperplasia . |
| FDBD01387 | Eltrombopag |

|
Thrombopoietic Agents; Blood and Blood Forming Organs; Antihemorrhagics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; | Thrombopoietin receptor agonists are pharmaceutical agents that stimulate platelet production in the bone marrow. In this, they differ from the previously discussed agents that act by attempting to curtail platelet destruction. |
| FDBD01400 | Udenafil |
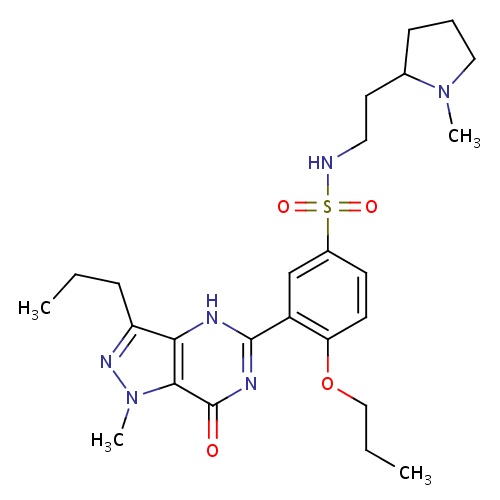
|
Genito Urinary System and Sex Hormones; Drugs Used in Erectile Dysfunction; Urological Agents; CYP3A4 Inhibitors; | Investigated for use/treatment in erectile dysfunction and hypertension. |
| FDBD01402 | Alvimopan |

|
Gastrointestinal Agents; Alimentary Tract and Metabolism; Drugs for Constipation; Peripheral Opioid Receptor Antagonists; | Used to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection surgery with primary anastomosis. Also investigated for use in the treatment of pain (acute or chronic). |
| FDBD01407 | Dapagliflozin |

|
sodium-glucose cotransporter 2 inhibitor ; Drugs Used in Diabetes; Alimentary Tract and Metabolism; Blood Glucose Lowering Drugs, Excl. Insulins; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Dapagliflozin is indicated for adjunct management of glycemic control in patients with type 2 diabetes mellitus, in combination with diet and exercise. |
261 ,
27
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4wr8_ligand_frag_0.mol2 | 4wr8 | 1 | -7.04 | c1(ccccc1)O | 7 |
| 4i7l_ligand_frag_0.mol2 | 4i7l | 1 | -7.03 | c1(ccccc1)O | 7 |
| 4i7l_ligand.mol2 | 4i7l | 1 | -7.03 | c1(ccccc1)O | 8 |
| 1mfi_ligand_frag_0.mol2 | 1mfi | 1 | -7.02 | c1ccc(cc1)O | 7 |
| 4wrb_ligand_frag_0.mol2 | 4wrb | 1 | -7.00 | c1(ccccc1)O | 7 |
| 1ljt_ligand_frag_3.mol2 | 1ljt | 1 | -6.98 | c1cc(ccc1)O | 7 |
| 2ra6_ligand_frag_1.mol2 | 2ra6 | 1 | -6.98 | c1ccc(cc1)O | 7 |
| 2ooz_ligand_frag_0.mol2 | 2ooz | 1 | -6.97 | c1(ccccc1)O | 7 |
| 1li2_ligand_frag_0.mol2 | 1li2 | 1 | -6.94 | c1(ccccc1)O | 7 |
1122 ,
113

