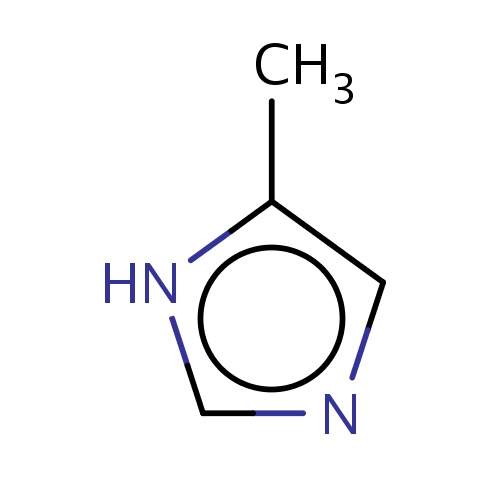
Common name
5-methyl-1H-imidazole
IUPAC name
5-methyl-1H-imidazole
SMILES
[nH]1c(cnc1)C
Common name
5-methyl-1H-imidazole
IUPAC name
5-methyl-1H-imidazole
SMILES
[nH]1c(cnc1)C
INCHI
InChI=1S/C4H6N2/c1-4-2-5-3-6-4/h2-3H,1H3,(H,5,6)
FORMULA
C4H6N2

Common name
5-methyl-1H-imidazole
IUPAC name
5-methyl-1H-imidazole
Molecular weight
82.104
clogP
1.559
clogS
-1.205
Frequency
0.0021
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
28.68
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00010 | L-Histidine |

|
Dietary Supplements; Micronutrients; Conditionally Essential Amino Acids; Supplements; | The actions of supplemental L-histidine are entirely unclear. It may have some immunomodulatory as well as antioxidant activity. L-histidine may be indicated for use in some with rheumatoid arthritis. It is not indicated for treatment of anemia or uremia or for lowering serum cholesterol. |
| FDBD00100 | Remikiren |
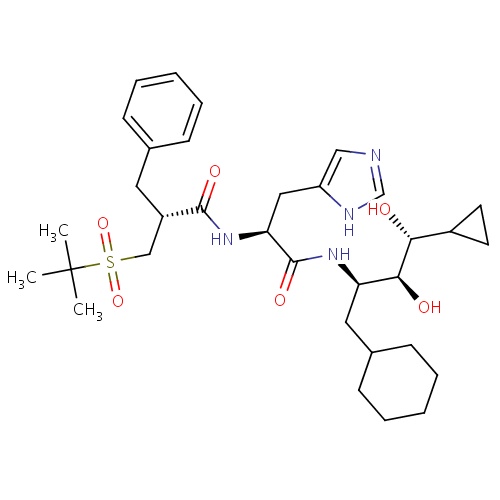
|
Cardiovascular System; Agents Acting on the Renin-Angiotensin System; Renin-Inhibitors; | For the treatment of hypertension and heart failure. |
| FDBD00374 | Cimetidine |
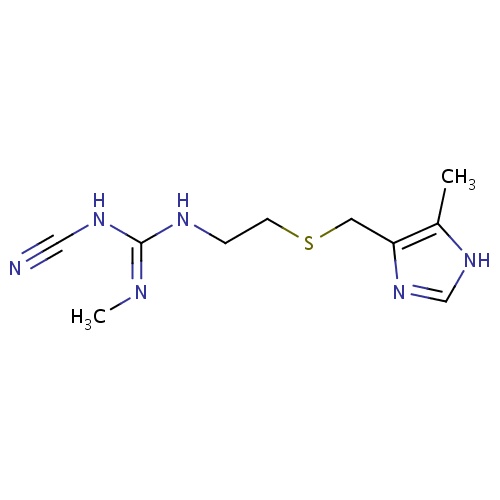
|
Anti-Ulcer Agents; Adjuvants; Alimentary Tract and Metabolism; Drugs for Peptic Ulcer and Gastro-Oesophageal Reflux Disease (Gord); Drugs for Acid Related Disorders; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; H2 Antagonists; BSEP/ABCB11 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment and the management of acid-reflux disorders (GERD), peptic ulcer disease, heartburn, and acid indigestion. |
| FDBD00823 | Alosetron |

|
Gastrointestinal Agents; Serotonin Antagonists; Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; Serotonin Receptor Antagonists; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Antiemetics Antagonists; | Only for the treatment of symptoms of severe diarrhea-predominant irritable bowel syndrome (IBS) in women with chronic symptoms (generally lasting greater than 6 months) who does not present with anatomic or biochemical GI abnormalities and have not responded to conventional therapy. |
| FDBD01336 | Nilotinib |
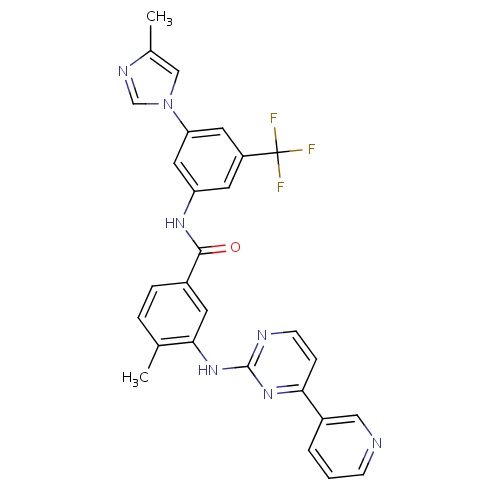
|
Antineoplastic Agents; Immunosuppressive Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the potential treatment of various leukemias, including chronic myeloid leukemia (CML). |
| FDBD01750 | Polaprezinc |
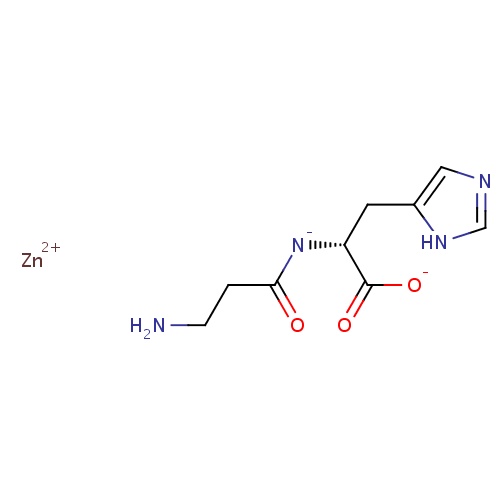
|
; |
6 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1qft_ligand_1_1.mol2 | 1qft | 1 | -6.25 | Cc1c[nH+]c[nH]1 | 6 |
| 1waw_ligand_1_5.mol2 | 1waw | 1 | -6.12 | Cc1c[nH+]c[nH]1 | 6 |
| 1w9u_ligand_1_6.mol2 | 1w9u | 1 | -6.09 | Cc1c[nH+]c[nH]1 | 6 |
| 3bu1_ligand_1_1.mol2 | 3bu1 | 1 | -6.08 | Cc1[nH+]c[nH]c1 | 6 |
| 2q2c_ligand_1_1.mol2 | 2q2c | 1 | -6.07 | c1([nH+]c[nH]c1)C | 6 |
| 1lag_ligand_1_1.mol2 | 1lag | 1 | -6.06 | c1(c[nH+]c[nH]1)C | 6 |
| 4pin_ligand_1_2.mol2 | 4pin | 1 | -6.02 | c1([nH+]c[nH]c1)C | 6 |
| 3atw_ligand_1_12.mol2 | 3atw | 1 | -6.00 | c1([nH+]c[nH]c1)C | 6 |
| 4tww_ligand_1_2.mol2 | 4tww | 1 | -6.00 | Cc1[nH+]c[nH]c1 | 6 |
104 ,
11

