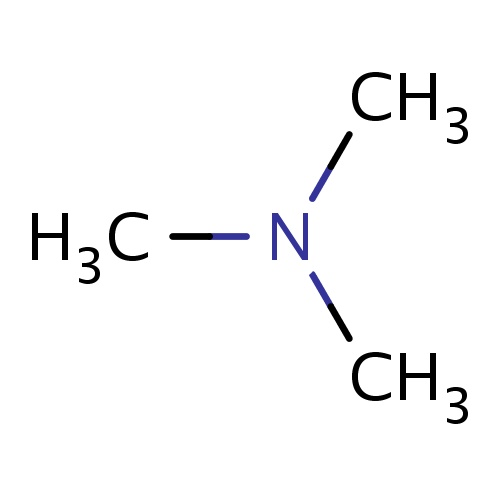
Common name
N,N-dimethylmethanamine
IUPAC name
N,N-dimethylmethanamine
SMILES
N(C)(C)C
Common name
N,N-dimethylmethanamine
IUPAC name
N,N-dimethylmethanamine
SMILES
N(C)(C)C
INCHI
InChI=1S/C3H9N/c1-4(2)3/h1-3H3
FORMULA
C3H9N

Common name
N,N-dimethylmethanamine
IUPAC name
N,N-dimethylmethanamine
Molecular weight
59.110
clogP
-0.577
clogS
-0.200
Frequency
0.0371
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01488 | Levopropoxyphene |
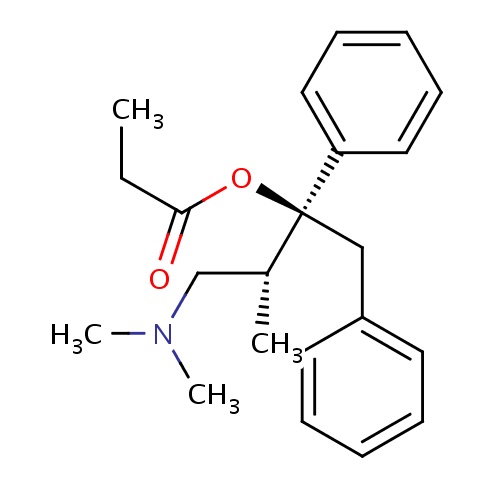
|
; | |
| FDBD01518 | Chloropyramine |

|
Histamine H1 Antagonists; Histamine Antagonists; Respiratory System; Dermatologicals; Antipruritics, Incl. Antihistamines, Anesthetics, Etc.; Antihistamines for Topical Use; Antihistamines for Systemic Use; Substituted Ethylene Diamines; | For the treatment of allergic conjunctivitis, allergic rhinitis, bronchial asthma, and other atopic (allergic) conditions. |
| FDBD01519 | Dimetindene |
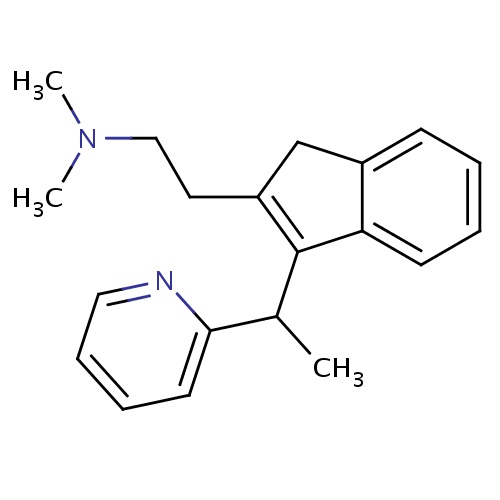
|
Anti-Allergic Agents; Antipruritics; Histamine H1 Antagonists; Cholinergic Antagonists; Histamine Antagonists; Respiratory System; Dermatologicals; Antipruritics, Incl. Antihistamines, Anesthetics, Etc.; Antihistamines for Topical Use; Antihistamines for Systemic Use; Substituted Alkylamines; | Indicated as symptomatic treatment of allergic reactions: urticaria, allergies of the upper respiratory tract such as hey fever and perennial rhinitis, food and drug allergies; pruritus of various origins, except pruritus due to cholestasis; insect bites. Dimethindene is also indicated for pruritus in eruptive skin diseases such as chicken-pox. Dimethindene can also be used as an adjuvant in eczema and other pruriginous dermatoses of allergic origin. |
| FDBD01541 | Acetylcarnitine |
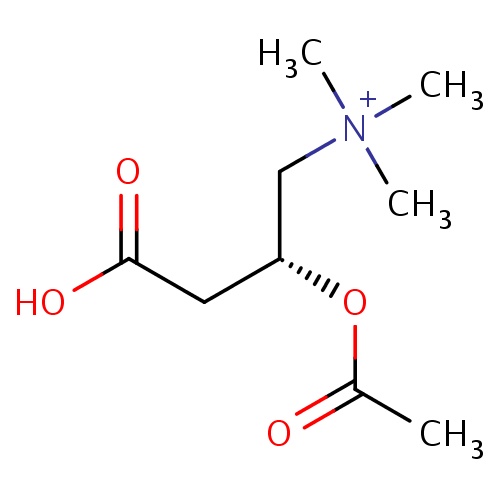
|
Acetylcarnitine is not approved for any indication in the United states and Canada, but it is approved and indicated in Italy for cerebrovascular disorders, mental function disorders, peripheral nerve disorders, diabetic neuropathy, and nutritional supplementation; Portugal for mental function disorders; Argentina for cerebral vasculopathy, nutritional supplementation, and peripheral neuropathy; Chile for dementia; Philippines for cerebrovascular disorders and mental function disorders; Australia for nutritional supplementation; and India for nutritional supplementation to increase sperm count. Acetylcarnitine also has several potential therapeutic indications for which it is still being investigated: in Norway, acetylcarnitine is in a phase IV trial for prophylactic treatment of migraines; in Italy acetylcarnitine is in a phase II trial for use in patients with type 2 Diabetes Mellitus, a phase III trial for alleviating fatigue in patients with chronic hepatitis C, and for use in patients with Minimal Hepatic Encephalopathy; in the United States acetylcarnitine is in a phase II trial for the neurodegenerative disorder Progressive Supranuclear Palsy, a phase II and III trial for reducing peripheral neuropathy in cancer patients as an adjunct to chemotherapy, a phase I and II trial for treating patients in septic shock, a phase II trial for bipolar depression, a phase II trial to reduce oxidative stress in patients with Sickle Cell disease, a phase I and II trial for chronic fatigue syndrome, and a study for preventing nerve damage in HIV patients; in China acetylcarnitine is in a phase III trial for reducing peripheral neuropathy in cancer patients as an adjunct to chemotherapy; in the United Kingdom acetylcarnitine is being investigated for preventing nerve damage in HIV patients; and in Israel acetylcarnitine is being studied for the treatment of male infertility. | |
| FDBD01565 | Bedaquiline |
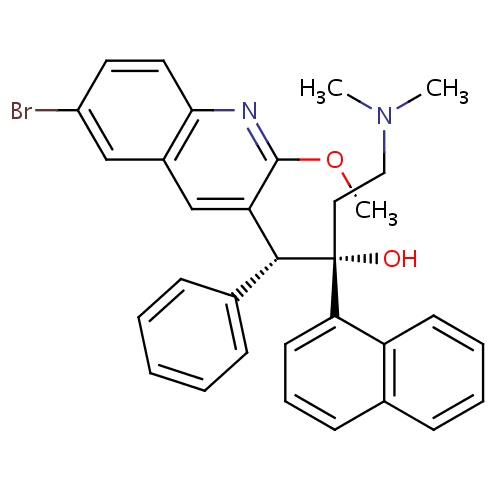
|
Antitubercular Agents; Antimycobacterials; Antiinfectives for Systemic Use; CYP3A4 Inhibitors; | Bedaquiline is indicated as part of combination therapy in adults ( |
| FDBD01575 | Afatinib |

|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; | Afatinib is a kinase inhibitor indicated for the first-line treatment of patient with metastatic non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. |
| FDBD01598 | Fominoben |

|
; | |
| FDBD01616 | Dimetacrine |

|
Nervous System; Antidepressants; Psychoanaleptics; Non-Selective Monoamine Reuptake Inhibitors; | |
| FDBD01618 | Cyamemazine |
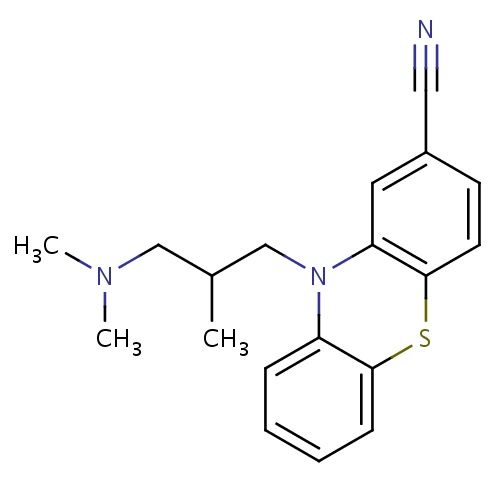
|
Antipsychotic Agents; Nervous System; Psycholeptics; Phenothiazines With Aliphatic Side-Chain; | |
| FDBD01625 | Captodiame |
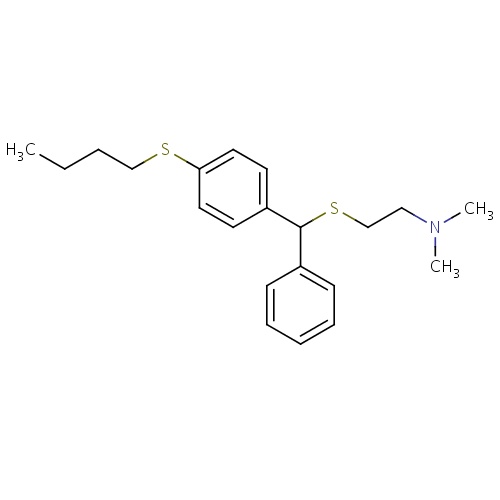
|
Nervous System; Anxiolytics; Psycholeptics; Diphenylmethane Derivatives; | Captodiame is indicated for the treatment of anxiety. |
108 ,
11
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2reg_ligand_1_1.mol2 | 2reg | 1 | -5.84 | C[N+](C)(C)C | 5 |
| 2rin_ligand_1_0.mol2 | 2rin | 1 | -5.74 | C[N+](C)(C)C | 5 |
| 4bgk_ligand_1_3.mol2 | 4bgk | 1 | -5.64 | [N+](C)(C)(C)C | 5 |
| 4c5w_ligand_1_0.mol2 | 4c5w | 1 | -5.64 | C[N+](C)(C)C | 5 |
| 1sw2_ligand_frag_0.mol2 | 1sw2 | 1 | -5.63 | [NH+](C)(C)C | 4 |
892 ,
90

