
Common name
methylurea
IUPAC name
methylurea
SMILES
O=C(NC)N
Common name
methylurea
IUPAC name
methylurea
SMILES
O=C(NC)N
INCHI
InChI=1S/C2H6N2O/c1-4-2(3)5/h1H3,(H3,3,4,5)
FORMULA
C2H6N2O

Common name
methylurea
IUPAC name
methylurea
Molecular weight
74.082
clogP
-1.216
clogS
0.225
Frequency
0.0065
HBond Acceptor
1
HBond Donor
3
Total PolarSurface Area
55.12
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00046 | L-Citrulline |
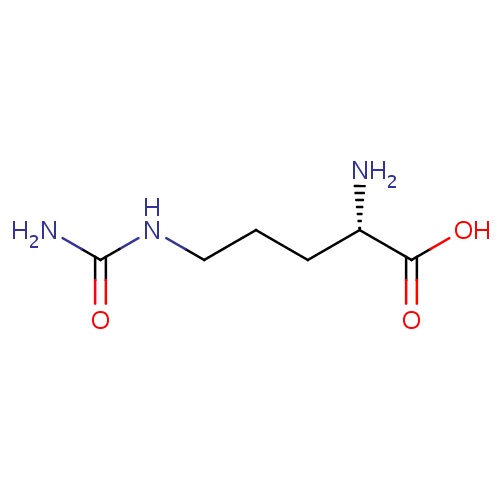
|
Dietary Supplements; Micronutrients; Supplements; Non-Essential Amino Acids; | Used for nutritional supplementation, also for treating dietary shortage or imbalance. |
| FDBD00150 | Carmustine |
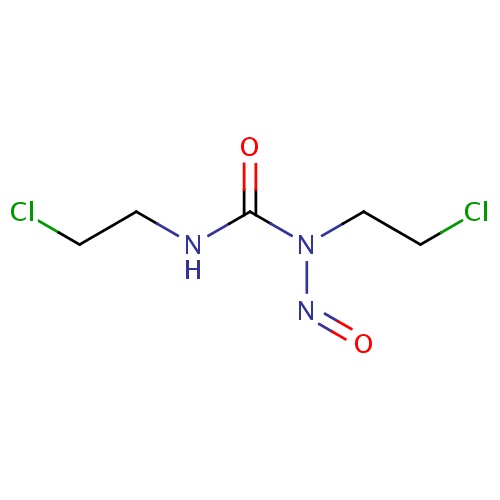
|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrosoureas; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; | For the treatment of brain tumors, multiple myeloma, Hodgkin's disease and Non-Hodgkin's lymphomas. |
| FDBD00305 | Streptozocin |
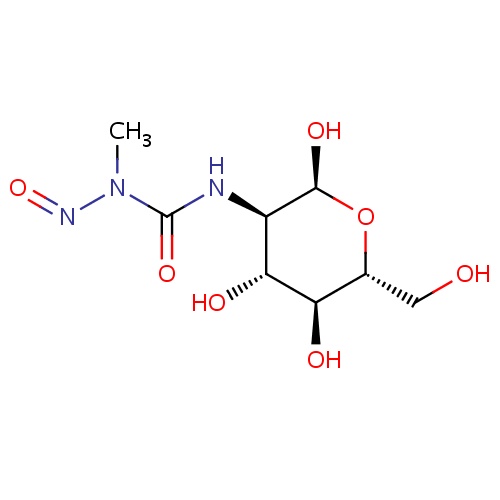
|
Antineoplastic Agents; Immunosuppressive Agents; Antibiotics, Antineoplastic; Alkylating Agents; Antibiotics; Antineoplastic and Immunomodulating Agents; Nitrosoureas; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); | For the treatment of malignant neoplasms of pancreas (metastatic islet cell carcinoma). |
| FDBD00376 | Ritonavir |

|
Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Combined Inducers of CYP3A4 and P-glycoprotein; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with other antiretroviral agents for the treatment of HIV-infection. |
| FDBD00401 | Chlormerodrin |

|
Diagnostic Agents; Diuretics, Mercurial; | Previously used as a diuretic. The radiolabeled form has been used as a diagnostic and research tool. |
| FDBD01052 | Lomustine |
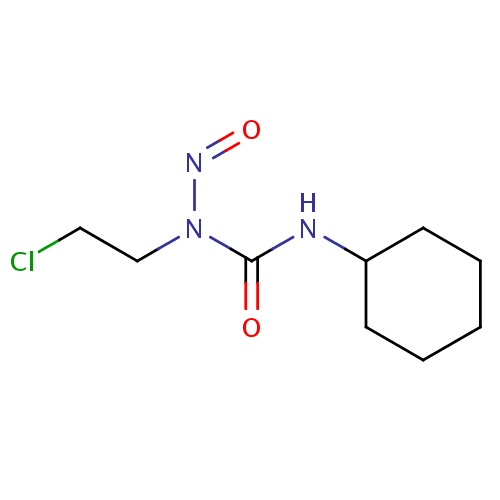
|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrosoureas; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of primary and metastatic brain tumors as a component of combination chemotherapy in addition to appropriate surgical and/or radiotherapeutic procedures. Also used in combination with other agents as secondary therapy for the treatment of refractory or relapsed Hodgkin's disease. |
| FDBD01550 | Boceprevir |
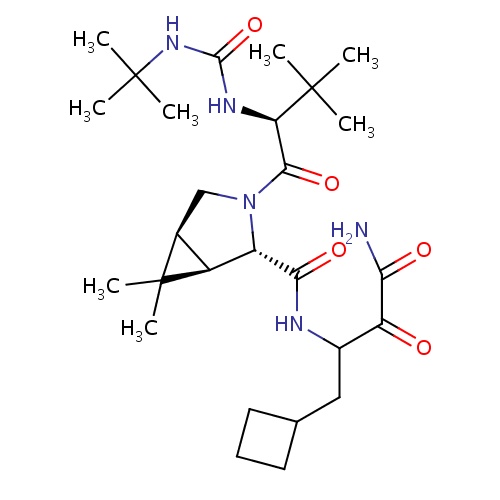
|
Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Treatment of chronic hepatitis C genotype 1 in patients that have a compensated liver (as a result of liver diseases like cirrhosis) and are previously untreated or therapy with peginterferon alfa and ribavirin has failed. |
| FDBD01647 | Ceftolozane |
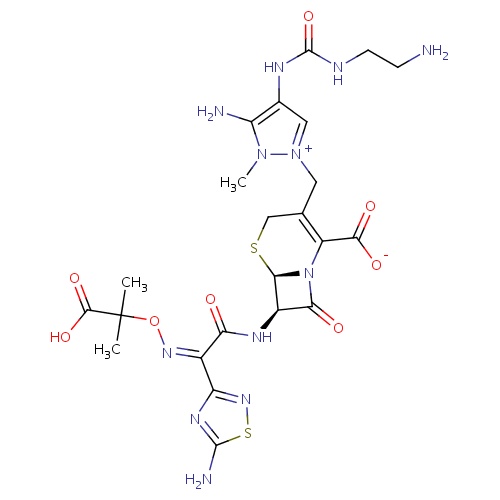
|
used in combination with metronidazole. | |
| FDBD01652 | Cobicistat |
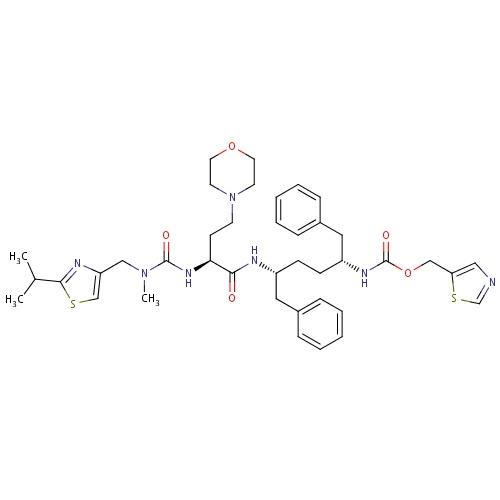
|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. |
| FDBD02476 | benzthiazuron |
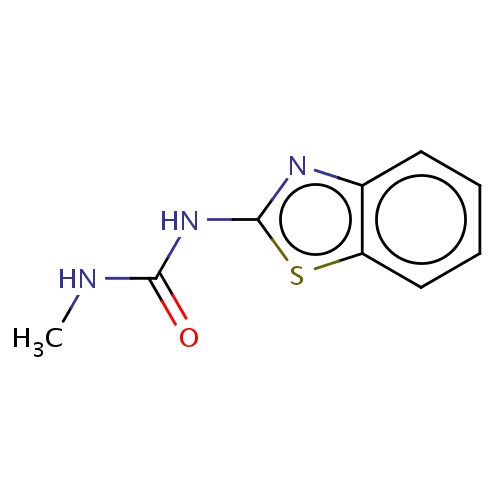
|
Herbicide | Herbicide |
19 ,
2
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1zd3_ligand_1_1.mol2 | 1zd3 | 1 | -5.79 | CNC(=O)N | 5 |
| 1zd4_ligand_1_1.mol2 | 1zd4 | 1 | -5.74 | NC(=O)NC | 5 |
| 5akz_ligand_frag_0.mol2 | 5akz | 1 | -5.73 | CNC(=O)N | 5 |
| 1zd5_ligand_1_1.mol2 | 1zd5 | 1 | -5.67 | NC(=O)NC | 5 |
| 2yix_ligand_1_1.mol2 | 2yix | 1 | -5.62 | NC(=O)NC | 5 |
| 5bvk_ligand_1_2.mol2 | 5bvk | 1 | -5.58 | CNC(=O)N | 5 |
| 3ipu_ligand_1_6.mol2 | 3ipu | 1 | -5.57 | N(C(=O)N)(C)C | 6 |
| 4uib_ligand_1_0.mol2 | 4uib | 1 | -5.57 | CNC(=O)N | 5 |
| 5bvn_ligand_1_1.mol2 | 5bvn | 1 | -5.57 | CNC(=O)N | 5 |
120 ,
13

