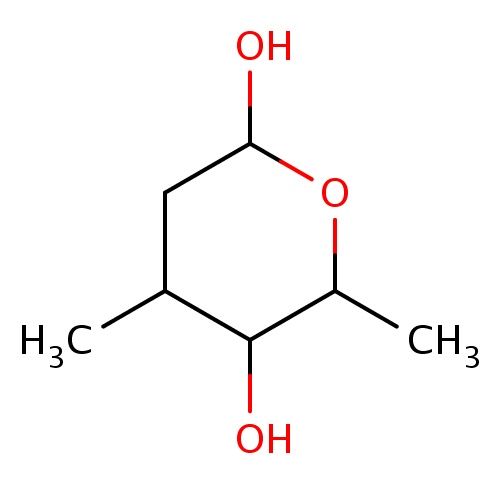
Common name
(2R,4S,5S,6R)-4,6-dimethyltetrahydropyran-2,5-diol
IUPAC name
(2R,4S,5S,6R)-4,6-dimethyltetrahydropyran-2,5-diol
SMILES
OC1OC(C(C(C1)C)O)C
Common name
(2R,4S,5S,6R)-4,6-dimethyltetrahydropyran-2,5-diol
IUPAC name
(2R,4S,5S,6R)-4,6-dimethyltetrahydropyran-2,5-diol
SMILES
OC1OC(C(C(C1)C)O)C
INCHI
InChI=1S/C7H14O3/c1-4-3-6(8)10-5(2)7(4)9/h4-9H,3H2,1-2H3/t4-,5+,6+,7-/m0/s1
FORMULA
C7H14O3

Common name
(2R,4S,5S,6R)-4,6-dimethyltetrahydropyran-2,5-diol
IUPAC name
(2R,4S,5S,6R)-4,6-dimethyltetrahydropyran-2,5-diol
Molecular weight
146.184
clogP
0.066
clogS
0.311
Frequency
0.0014
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
49.69
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00087 | Erythromycin |

|
Gastrointestinal Agents; Enzyme Inhibitors; Anti-Bacterial Agents; Protein Synthesis Inhibitors; Macrolides; Anti-Acne Preparations; Antibiotics; Ophthalmologicals; Sensory Organs; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Dermatologicals; Anti-Acne Preparations for Topical Use; Antiinfectives; Antiinfectives for Treatment of Acne; Macrolides, Lincosamides and Streptogramins; Macrolides and Lincosamides for Intramammary Use; Antibacterials for Intramammary Use; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For use in the treatment of infections caused by susceptible strains of microorganisms in the following diseases: respiratory tract infections (upper and lower) of mild to moderate degree, pertussis (whooping cough), as adjunct to antitoxin in infections due to . |
| FDBD00095 | Azithromycin |
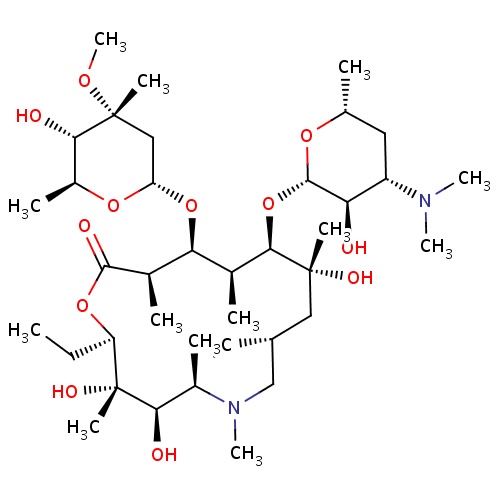
|
Anti-Bacterial Agents; Macrolides; Antibiotics; Ophthalmologicals; Sensory Organs; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Antiinfectives; Macrolides, Lincosamides and Streptogramins; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions: . |
| FDBD00641 | Roxithromycin |

|
Anti-Bacterial Agents; Macrolides; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Macrolides, Lincosamides and Streptogramins; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | Used to treat respiratory tract, urinary and soft tissue infections. |
| FDBD01057 | Clarithromycin |

|
Anti-Bacterial Agents; Protein Synthesis Inhibitors; Macrolides; Alimentary Tract and Metabolism; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Macrolides, Lincosamides and Streptogramins; Drugs for Peptic Ulcer and Gastro-Oesophageal Reflux Disease (Gord); Drugs for Acid Related Disorders; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C19 Inducers; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | An alternative medication for the treatment of acute otitis media caused by . |
4 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2x6w_ligand_1_13.mol2 | 2x6w | 1 | -6.56 | C[C@@H]1[C@H](CC[C@H](O1)O)O | 9 |
| 1m7d_ligand_1_6.mol2 | 1m7d | 1 | -6.46 | O[C@H]1CC[C@H]([C@@H](O1)C)O | 9 |
| 2x85_ligand_1_13.mol2 | 2x85 | 1 | -6.46 | C[C@@H]1[C@H](CC[C@@H](O1)O)O | 9 |
| 2x6y_ligand_1_13.mol2 | 2x6y | 1 | -6.24 | [C@@H]1(CC[C@@H]([C@H](O1)C)O)O | 9 |
| 2iyf_ligand_1_3.mol2 | 2iyf | 1 | -5.40 | [C@@H]1(C[C@@H]([C@H]([C@@H](O1)C)O)C)O | 10 |
| 4oeg_ligand_2_8.mol2 | 4oeg | 1 | -5.15 | C[C@@H]1[C@H](CC[C@H](O1)O)O | 9 |
| 4urn_ligand_1_2.mol2 | 4urn | 0.96875 | -6.41 | O[C@H]1[C@@H](CCC(O1)(C)C)O | 10 |
| 4uro_ligand_1_2.mol2 | 4uro | 0.96875 | -6.37 | C1C[C@H]([C@@H](OC1(C)C)O)O | 10 |
| 1kzn_ligand_1_2.mol2 | 1kzn | 0.96875 | -6.31 | C1C(O[C@H]([C@@H](C1)O)O)(C)C | 10 |
106 ,
11

