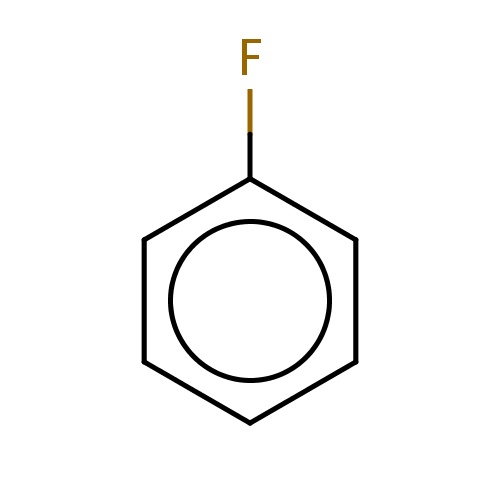
Common name
fluorobenzene
IUPAC name
fluorobenzene
SMILES
Fc1ccccc1
Common name
fluorobenzene
IUPAC name
fluorobenzene
SMILES
Fc1ccccc1
INCHI
InChI=1S/C6H5F/c7-6-4-2-1-3-5-6/h1-5H
FORMULA
C6H5F

Common name
fluorobenzene
IUPAC name
fluorobenzene
Molecular weight
96.102
clogP
2.398
clogS
-1.833
Frequency
0.0237
HBond Acceptor
0
HBond Donor
0
Total PolarSurface Area
0
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01374 | Sertindole |

|
Antipsychotic Agents; Nervous System; Psycholeptics; Indole Derivatives; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Used in the treatment of schizophrenia. |
| FDBD01386 | Prasugrel |

|
Platelet Aggregation Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | Indicated in combination with acetylsalicylic acid (ASA) to prevent atherothrombotic events in patients with acute coronary syndrome (ACS) who are to be managed with percutaneous coronary intervention (PCI). May be used in patients with unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI), ST-elevation myocardial infarction (STEMI) who are to be managed with PCI. Prasugrel is not recommended in patients 75 years of age or greater, those that weigh. |
| FDBD01426 | Flupirtine |
|
Analgesics; Nervous System; | Investigated for use/treatment in fibromyalgia. |
| FDBD01457 | Fosaprepitant |

|
Antiemetics; Neurokinin-1 Receptor Antagonists; | For the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy. |
| FDBD01502 | Raltegravir |

|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; HIV Integrase Inhibitors; | For the treatment of HIV-1 infection in conjunction with other antiretrovirals. |
| FDBD01543 | Pitavastatin |
|
Hydroxymethylglutaryl-CoA Reductase Inhibitors; Hypolipidemic Agents; HMG CoA Reductase Inhibitors; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; | Pitavastatin is used to lower serum levels of total cholesterol, LDL-C, apolipoprotein B, and triglycerides, and raise levels of HDL-C for the treatment of dyslipidemia. |
| FDBD01561 | Regorafenib |
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Regorafenib is indicated for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy. Regorafenib is also indicated for the treatment of patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumor (GIST) who have been previously treated with imatinib mesylate and sunitinib malate. |
| FDBD01563 | Enzalutamide |
|
Antineoplastic Agents; Antineoplastic and Immunomodulating Agents; Endocrine Therapy; Hormone Antagonists and Related Agents; Anti-Androgens; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Enzalutamide is indicated for the treatment of patients with metastatic castration-resistant prostate cancer who have previously received docetaxel. |
| FDBD01568 | Canagliflozin |

|
Hypoglycemic Agents; Drugs Used in Diabetes; Alimentary Tract and Metabolism; Blood Glucose Lowering Drugs, Excl. Insulins; CYP3A4 Inhibitors; | Canagliflozin is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Use in type 1 diabetes mellitus patients or in treatment of diabetic ketoacidosis is not recommended. |
| FDBD01573 | Dabrafenib |

|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | Dabrafenib is indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test. |
69 ,
7
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1xkk_ligand_frag_10.mol2 | 1xkk | 1 | -6.81 | c1cccc(F)c1 | 7 |
| 2rgp_ligand_frag_0.mol2 | 2rgp | 1 | -6.79 | c1ccccc1F | 7 |
| 3bel_ligand_frag_0.mol2 | 3bel | 1 | -6.78 | c1cc(ccc1)F | 7 |
| 2r4b_ligand_frag_0.mol2 | 2r4b | 1 | -6.77 | Fc1ccccc1 | 7 |
| 3fu0_ligand_frag_0.mol2 | 3fu0 | 1 | -6.77 | Fc1ccccc1 | 7 |
| 4l8m_ligand_frag_2.mol2 | 4l8m | 1 | -6.77 | Fc1ccccc1 | 7 |
| 1zz2_ligand_frag_5.mol2 | 1zz2 | 1 | -6.74 | c1ccc(cc1)F | 7 |
| 3bbt_ligand_frag_5.mol2 | 3bbt | 1 | -6.74 | c1cccc(F)c1 | 7 |
443 ,
45

