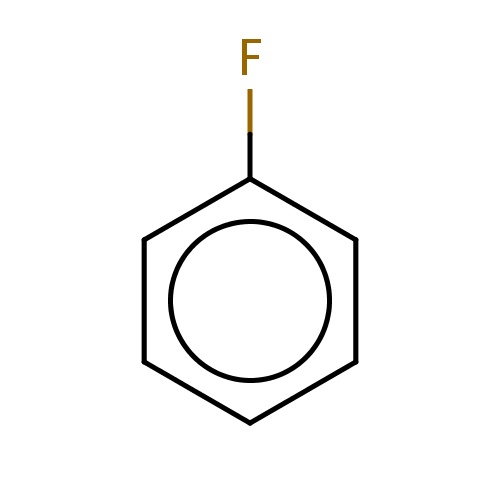
Common name
fluorobenzene
IUPAC name
fluorobenzene
SMILES
Fc1ccccc1
Common name
fluorobenzene
IUPAC name
fluorobenzene
SMILES
Fc1ccccc1
INCHI
InChI=1S/C6H5F/c7-6-4-2-1-3-5-6/h1-5H
FORMULA
C6H5F

Common name
fluorobenzene
IUPAC name
fluorobenzene
Molecular weight
96.102
clogP
2.398
clogS
-1.833
Frequency
0.0237
HBond Acceptor
0
HBond Donor
0
Total PolarSurface Area
0
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00579 | Paroxetine |

|
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors; Antidepressive Agents; Nervous System; Antidepressants; Psychoanaleptics; Selective Serotonin Reuptake Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); | Labeled indications include: major depressive disorder (MDD), panic disorder with or without agoraphobia, obsessive-compulsive disorder (OCD), social anxiety disorder (social phobia), generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), and premenstrual dysphoric disorder (PMDD). Unlabeled indications include: eating disorders, impulse control disorders, vasomotor symptoms of menopause, obsessive-compulsive disorder (OCD) in children, and mild dementia-associated agitation in nonpsychotic individuals. Brisdelle, which consists of paroxetine mesylate is indicated for the treatment of moderate to severe vasomotor symptoms (like hot flashes) associated with menopause. |
| FDBD00827 | Ezetimibe |

|
Anticholesteremic Agents; Cholesterol Absorption Inhibitors; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; CYP3A4 Inhibitors; | For use as adjunctive therapy to diet for the reduction of elevated total-C, LDL-C, and Apo B in patients with primary (heterozygous familial and non-familial) hypercholesterolemia. |
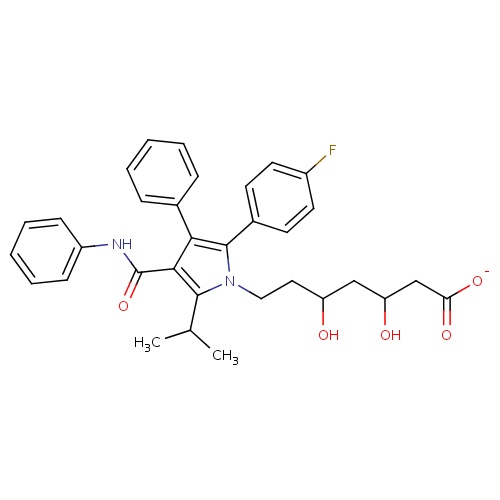
| FDBD00927 | Atorvastatin |
|
Anticholesteremic Agents; Hydroxymethylglutaryl-CoA Reductase Inhibitors; Dipeptidyl-Peptidase IV Inhibitors; HMG CoA Reductase Inhibitors; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | |
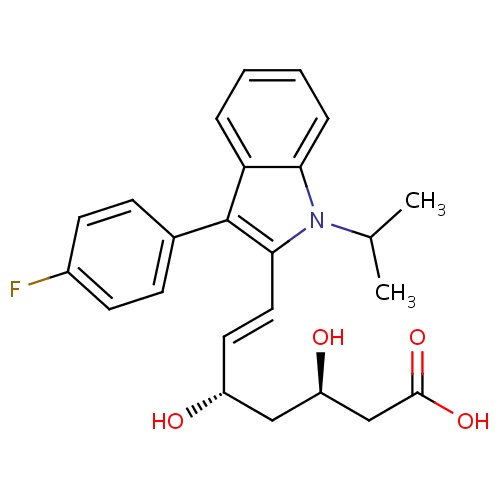
| FDBD00945 | Fluvastatin |
|
Anticholesteremic Agents; Hydroxymethylglutaryl-CoA Reductase Inhibitors; HMG CoA Reductase Inhibitors; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | To be used as an adjunct to dietary therapy to prevent cardiovascular events. May be used as secondary prevention in patients with coronary heart disease (CHD) to reduce the risk of requiring coronary revascularization procedures, for reducing progression of coronary atherosclerosis in hypercholesterolemic patients with CHD, and for the treatment of primary hypercholesterolemia and mixed dyslidipidemia. |
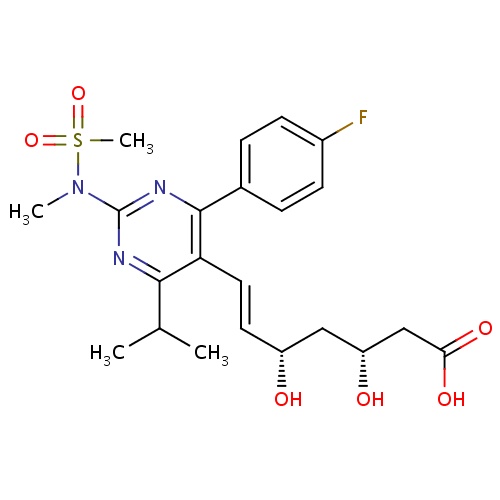
| FDBD00948 | Rosuvastatin |
|
Hydroxymethylglutaryl-CoA Reductase Inhibitors; HMG CoA Reductase Inhibitors; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP3A4 Inhibitors; | Used as an adjunct to dietary therapy to treat primary hyperlipidemia (heterozygous familial and nonfamilial), mixed dyslipidemia and hypertriglyceridemia. Also indicated for homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering therapies or when other such therapies are not available. Furthermore, it is used to slow the progression of atherosclerosis and for primary prevention of cardiovascular disease. |
| FDBD00950 | Pimozide |
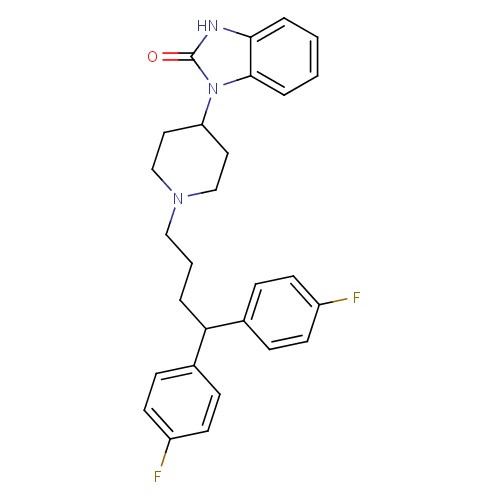
|
Antipsychotic Agents; Dopamine Antagonists; Anti-Dyskinesia Agents; Nervous System; Psycholeptics; Diphenylbutylpiperidine Derivatives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Used for the suppression of motor and phonic tics in patients with Tourette's Disorder who have failed to respond satisfactorily to standard treatment. |
| FDBD00956 | Levocabastine |

|
Histamine H1 Antagonists, Non-Sedating; Respiratory System; Ophthalmologicals; Sensory Organs; Nasal Preparations; Decongestants and Antiallergics; Antiallergic Agents, Excl. Corticosteroids; | As an ophthalmic for the temporary relief of the signs and symptoms of seasonal allergic conjunctivitis. Also used as a nasal spray for allergic rhinitis. |
| FDBD00977 | Bicalutamide |
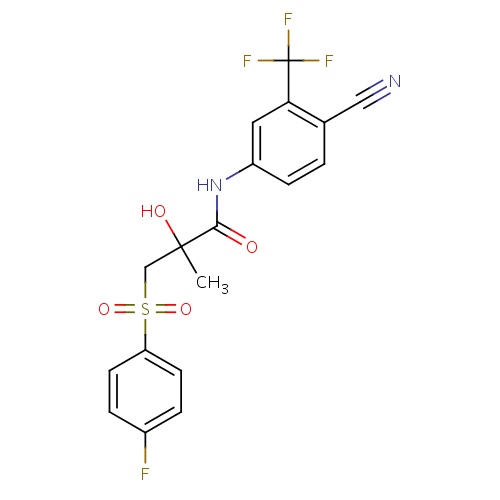
|
Antineoplastic and Immunomodulating Agents; Endocrine Therapy; Hormone Antagonists and Related Agents; Anti-Androgens; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For treatment (together with surgery or LHRH analogue) of advanced prostatic cancer. |
| FDBD01021 | Escitalopram |
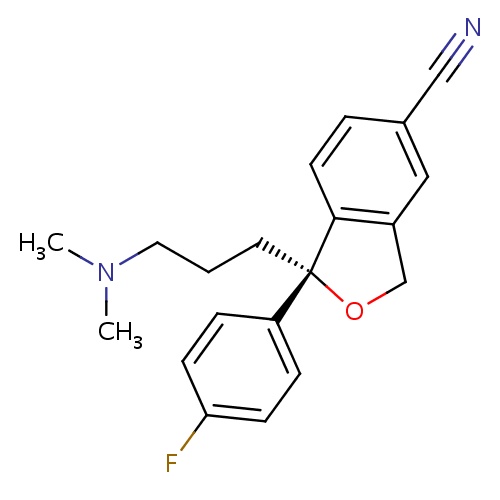
|
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors; Adrenergic alpha-1 Receptor Antagonists; Nervous System; Antidepressants; Psychoanaleptics; Selective Serotonin Reuptake Inhibitors; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Labeled indications include major depressive disorder (MDD) and generalized anxiety disorder (GAD). Unlabeled indications include treatment of mild dementia-associated agitation in nonpsychotic patients. |
| FDBD01101 | Lapatinib |
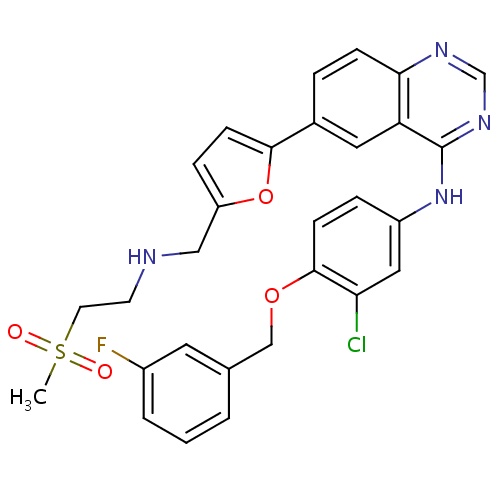
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress the human epidermal receptor type 2 (HER2) protein and who have received prior therapy including an anthracycline, a taxane, and trastuzuma. |
69 ,
7
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1xkk_ligand_frag_10.mol2 | 1xkk | 1 | -6.81 | c1cccc(F)c1 | 7 |
| 2rgp_ligand_frag_0.mol2 | 2rgp | 1 | -6.79 | c1ccccc1F | 7 |
| 3bel_ligand_frag_0.mol2 | 3bel | 1 | -6.78 | c1cc(ccc1)F | 7 |
| 2r4b_ligand_frag_0.mol2 | 2r4b | 1 | -6.77 | Fc1ccccc1 | 7 |
| 3fu0_ligand_frag_0.mol2 | 3fu0 | 1 | -6.77 | Fc1ccccc1 | 7 |
| 4l8m_ligand_frag_2.mol2 | 4l8m | 1 | -6.77 | Fc1ccccc1 | 7 |
| 1zz2_ligand_frag_5.mol2 | 1zz2 | 1 | -6.74 | c1ccc(cc1)F | 7 |
| 3bbt_ligand_frag_5.mol2 | 3bbt | 1 | -6.74 | c1cccc(F)c1 | 7 |
443 ,
45

