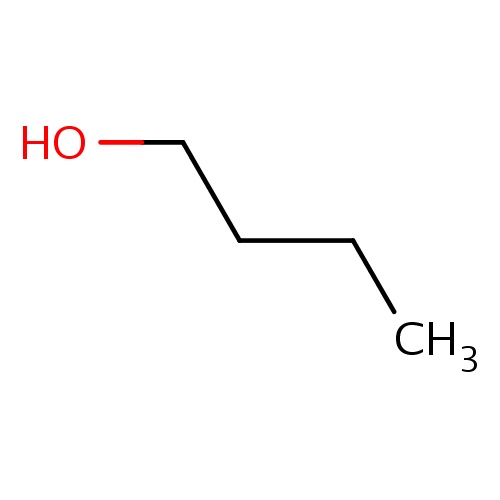
Common name
butan-1-ol
IUPAC name
butan-1-ol
SMILES
C(CC)CO
Common name
butan-1-ol
IUPAC name
butan-1-ol
SMILES
C(CC)CO
INCHI
InChI=1S/C4H10O/c1-2-3-4-5/h5H,2-4H2,1H3
FORMULA
C4H10O

Common name
butan-1-ol
IUPAC name
butan-1-ol
Molecular weight
74.122
clogP
0.437
clogS
-0.772
Frequency
0.0134
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
20.23
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01012 | Cilostazol |
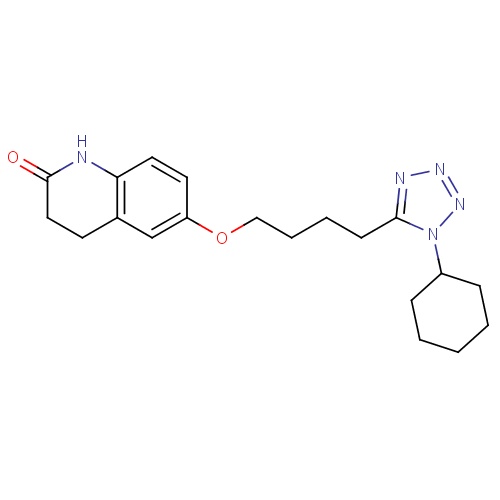
|
Fibrinolytic Agents; Platelet Aggregation Inhibitors; Bronchodilator Agents; Phosphodiesterase 3 Inhibitors; Vasodilator Agents; Neuroprotective Agents; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the reduction of symptoms of intermittent claudication (pain in the legs that occurs with walking and disappears with rest). |
| FDBD01075 | Diphenidol |
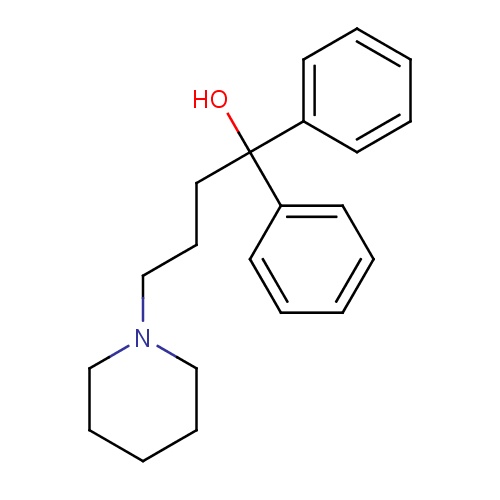
|
Antiemetics; | For use in the prevention and symptomatic treatment of peripheral (labyrinthine) vertigo and associated nausea and vomiting that occur in such conditions as Meniere's disease and surgery of the middle and inner ear. Also for the control of nausea and vomiting associated with postoperative states, malignant neoplasms, labyrinthine disturbances, antineoplastic agent therapy, radiation sickness, and infectious diseases. |
| FDBD01082 | Aripiprazole |
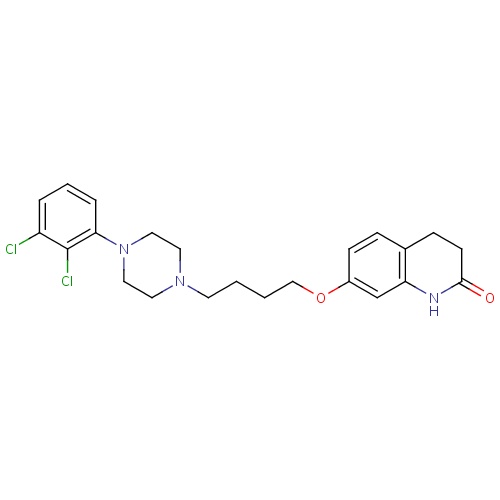
|
Antipsychotic Agents; Adrenergic alpha-1 Receptor Antagonists; Nervous System; Psycholeptics; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Antiemetics Antagonists; | For the treatment of schizophrenia and related psychotic disorders. |
| FDBD01084 | Epoprostenol |
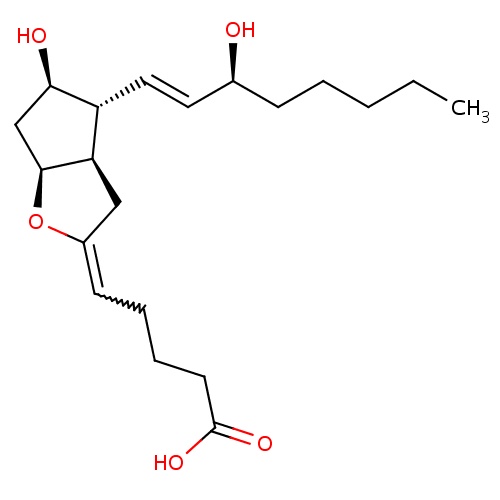
|
Platelet Aggregation Inhibitors; Antihypertensive Agents; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; | For the long-term intravenous treatment of primary pulmonary hypertension and pulmonary hypertension associated with the scleroderma spectrum of disease in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy. |
| FDBD01162 | Procaterol |
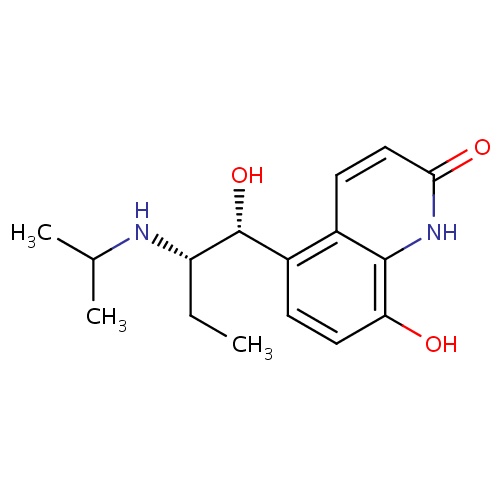
|
Sympathomimetics; Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Adrenergics for Systemic Use; Beta2 Agonists; | For the treatment of asthma and chronic obstructive pulmonary disease (COPD). |
| FDBD01187 | Pranlukast |
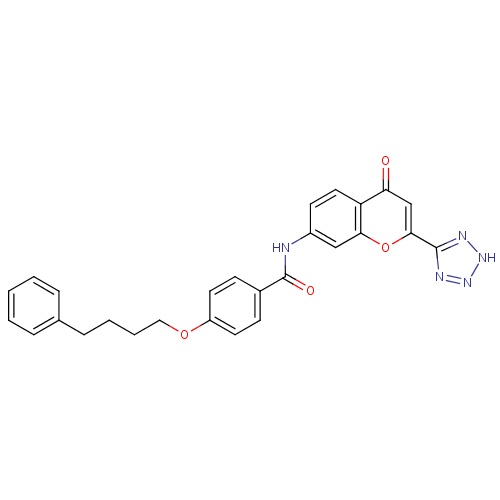
|
Anti-Asthmatic Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Leukotriene Receptor Antagonists; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP3A4 Inhibitors; | Used as an adjunct to the standard therapy of inhaled steroids with inhaled long- and/or short-acting beta-agonists. |
| FDBD01251 | Lopinavir |
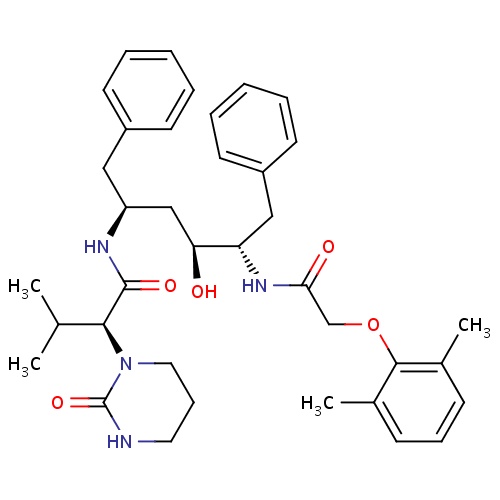
|
Anti-HIV Agents; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with other antiretroviral agents for the treatment of HIV-infection. |
| FDBD01664 | Idebenone |

|
Nervous System; Psychoanaleptics; Psychostimulants, Agents Used for Adhd and Nootropics; | |
| FDBD01665 | Vilanterol |
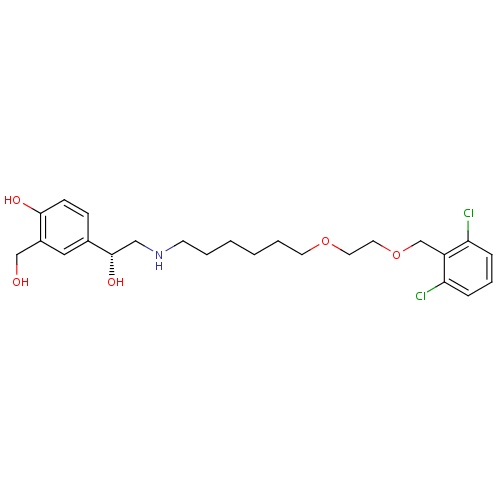
|
Immunosuppressive Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Adrenergics, Inhalants; CYP3A4 Inhibitors; Beta2 Agonists; | Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename Breo Ellipta and in combination with umeclidinium bromide as Anoro Ellipta. Approved by the FDA in 2013, use of Breo Ellipta is indicated for the long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema. It is also indicated for once-daily maintenance treatment of asthma in patients aged 18 or older with reversible obstructive airways disease. |
| FDBD01697 | Brexpiprazole |
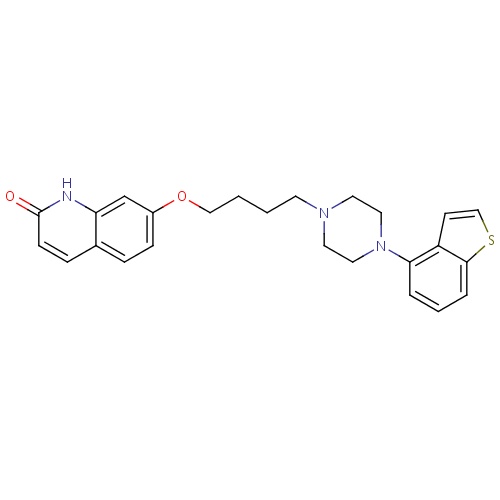
|
Antipsychotic Agents; Dopamine Agonists; Adrenergic alpha-1 Receptor Antagonists; Serotonin Agents; Nervous System; Psycholeptics; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | As an adjunctive treatment of major depressive disorder (MDD) and for treatment of schizophrenia. |
39 ,
4
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1esz_ligand_3_28.mol2 | 1esz | 1 | -6.36 | C(C)(CCO)C | 6 |
| 1fbm_ligand_3_19.mol2 | 1fbm | 1 | -6.22 | C(O)CC(C)C | 6 |
| 4f7n_ligand_3_25.mol2 | 4f7n | 1 | -6.13 | C(O)CCC | 5 |
| 1esz_ligand_2_9.mol2 | 1esz | 1 | -6.05 | C(C)CCO | 5 |
| 3egt_ligand_4_315.mol2 | 3egt | 1 | -5.99 | CCCCO | 5 |
| 4h5e_ligand_3_19.mol2 | 4h5e | 1 | -5.99 | OCCC(C)C | 6 |
| 3dzt_ligand_3_235.mol2 | 3dzt | 1 | -5.98 | CCCCO | 5 |
269 ,
27

