
Common name
N-[(1S)-1-(2,4-dichlorophenyl)ethyl]hydroxylamine
IUPAC name
N-[(1S)-1-(2,4-dichlorophenyl)ethyl]hydroxylamine
SMILES
Clc1c(ccc(c1)Cl)C(NO)C
Common name
N-[(1S)-1-(2,4-dichlorophenyl)ethyl]hydroxylamine
IUPAC name
N-[(1S)-1-(2,4-dichlorophenyl)ethyl]hydroxylamine
SMILES
Clc1c(ccc(c1)Cl)C(NO)C
INCHI
InChI=1S/C8H9Cl2NO/c1-5(11-12)7-3-2-6(9)4-8(7)10/h2-5,11-12H,1H3
FORMULA
C8H9Cl2NO

Common name
N-[(1S)-1-(2,4-dichlorophenyl)ethyl]hydroxylamine
IUPAC name
N-[(1S)-1-(2,4-dichlorophenyl)ethyl]hydroxylamine
Molecular weight
206.069
clogP
1.726
clogS
-3.094
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
20.23
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00127 | Oxiconazole |
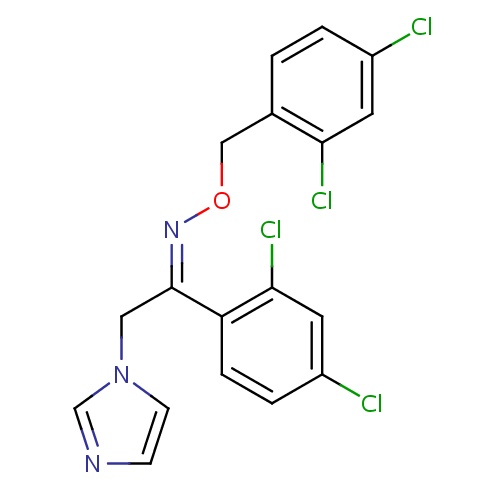
|
Antifungal Agents; Genito Urinary System and Sex Hormones; Dermatologicals; Gynecological Antiinfectives and Antiseptics; Imidazole and Triazole Derivatives; Antifungals for Topical Use; Antifungals for Dermatological Use; Imidazole Derivatives; Cytochrome P-450 CYP2C19 Inducers; CYP3A4 Inhibitors; | For treatment of dermal fungal infection. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1vru_ligand_2_4.mol2 | 1vru | 0.644444 | -7.14 | c1(c(cccc1Cl)Cl)C[NH3+] | 10 |
| 4mm9_ligand_3_100.mol2 | 4mm9 | 0.644444 | -7.14 | O[NH2+][C@H](C)c1ccccc1 | 10 |
| 1nhv_ligand_1_3.mol2 | 1nhv | 0.603774 | -6.97 | c1(ccc(cc1Cl)Cl)C(=O)N | 11 |
| 1nhu_ligand_1_3.mol2 | 1nhu | 0.603774 | -6.91 | NC(=O)c1ccc(cc1Cl)Cl | 11 |
| 2q7q_ligand.mol2 | 2q7q | 0.6 | -7.15 | [NH3+]Cc1ccc(cc1)Cl | 10 |
| 4cl6_ligand_2_0.mol2 | 4cl6 | 0.6 | -6.96 | Clc1ccc(cc1)C[NH3+] | 9 |
| 3jzi_ligand_2_14.mol2 | 3jzi | 0.6 | -6.86 | [NH3+]Cc1ccccc1Cl | 9 |
| 3jzf_ligand_2_14.mol2 | 3jzf | 0.6 | -6.83 | c1(ccccc1Cl)C[NH3+] | 9 |
| 4yes_ligand_1_3.mol2 | 4yes | 0.6 | -6.82 | C([NH3+])c1ccc(cc1)Cl | 9 |
100 ,
11

