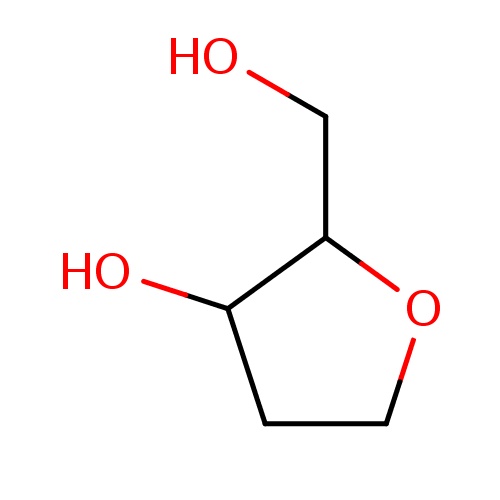
Common name
(2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol
IUPAC name
(2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol
SMILES
C(O)C1OCCC1O
Common name
(2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol
IUPAC name
(2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol
SMILES
C(O)C1OCCC1O
INCHI
InChI=1S/C5H10O3/c6-3-5-4(7)1-2-8-5/h4-7H,1-3H2/t4-,5+/m0/s1
FORMULA
C5H10O3

Common name
(2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol
IUPAC name
(2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol
Molecular weight
118.131
clogP
0.099
clogS
0.528
Frequency
0.0021
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
49.69
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
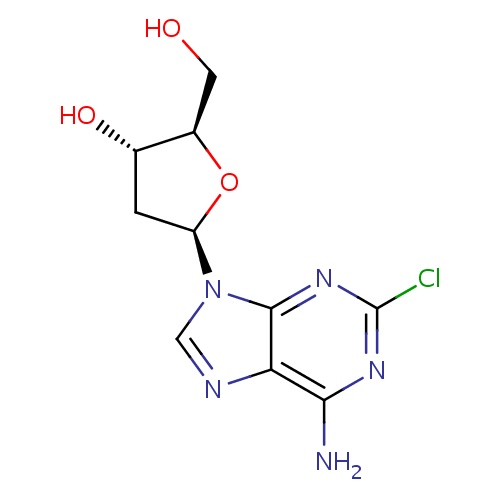
| FDBD00130 | Cladribine |
|
Antineoplastic Agents; Immunosuppressive Agents; Antimetabolites; Purine analogues; Antineoplastic and Immunomodulating Agents; | For the treatment of active hairy cell leukemia (leukemic reticuloendotheliosis) as defined by clinically significant anemia, neutropenia, thrombocytopenia, or disease-related symptoms. Also used as an alternative agent for the treatment of chronic lymphocytic leukemia (CLL), low-grade non-Hodgkin's lymphoma, and cutaneous T-cell lymphoma. |
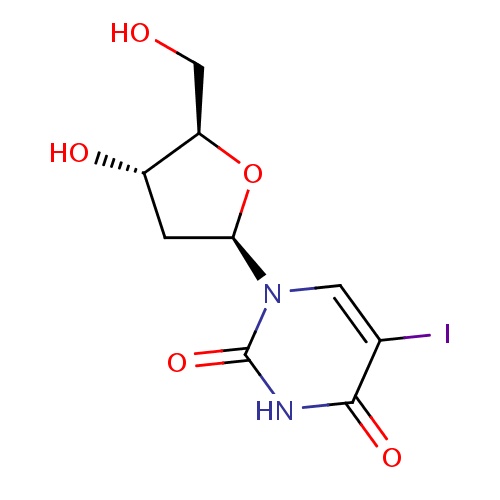
| FDBD00137 | Idoxuridine |
|
Antiviral Agents; Nucleic Acid Synthesis Inhibitors; Ophthalmologicals; Sensory Organs; Antiinfectives for Systemic Use; Dermatologicals; Direct Acting Antivirals; Antivirals for Systemic Use; Nucleosides and Nucleotides Excl. Reverse Transcriptase Inhibitors; Antiinfectives; | For use in keratoconjunctivitis and keratitis caused by herpes simplex virus. |
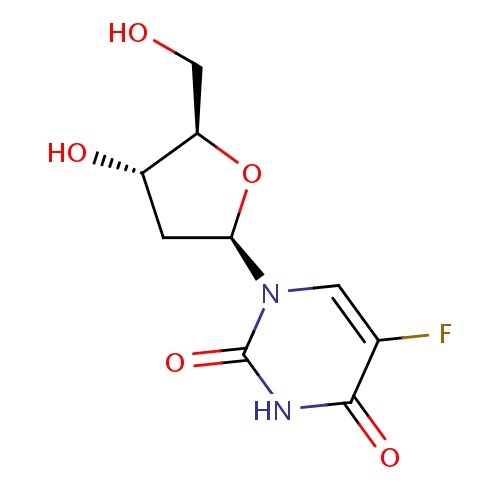
| FDBD00208 | Floxuridine |
|
Antineoplastic Agents; Immunosuppressive Agents; Antimetabolites, Antineoplastic; | For palliative management of gastrointestinal adenocarcinoma metastatic to the liver, when given by continuous regional intra-arterial infusion in carefully selected patients who are considered incurable by surgery or other means. Also for the palliative management of liver cancer (usually administered by hepatic intra-arterial infusion). |
| FDBD00309 | Trifluridine |
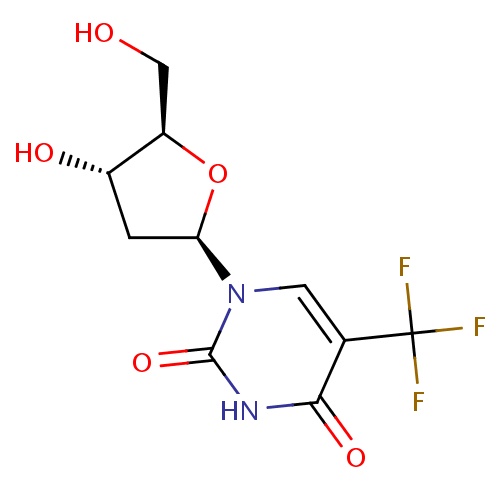
|
Antineoplastic Agents; Antiviral Agents; Antimetabolites; Antineoplastic and Immunomodulating Agents; Ophthalmologicals; Sensory Organs; Antiinfectives; Pyrimidine Analogues; | Ophthalmic solution for the treatment of primay keratoconjunctivitis and recurrent epithelial keratitis due to herpes simplex virus, types 1 and 2. |
| FDBD00419 | Pentostatin |

|
Antineoplastic Agents; Immunosuppressive Agents; Adenosine Deaminase Inhibitors; Antibiotics; Antineoplastic and Immunomodulating Agents; | For the treatment of hairy cell leukaemia refractory to alpha interferon. |
| FDBD01104 | Decitabine |

|
Antineoplastic Agents; Enzyme Inhibitors; Antimetabolites; Antimetabolites, Antineoplastic; Teratogens; Antineoplastic and Immunomodulating Agents; Pyrimidine Analogues; | For treatment of patients with myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups (scores |
6 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2py4_ligand_2_6.mol2 | 2py4 | 1 | -6.37 | C1C[C@@H]([C@H](O1)CO)O | 8 |
| 3ehw_ligand_2_6.mol2 | 3ehw | 1 | -6.35 | OC[C@@H]1[C@H](CCO1)O | 8 |
| 2yay_ligand_2_6.mol2 | 2yay | 1 | -6.25 | C1C[C@@H]([C@H](O1)CO)O | 8 |
| 2yb0_ligand_1_1.mol2 | 2yb0 | 1 | -6.25 | C1C[C@@H]([C@H](O1)CO)O | 8 |
| 4uxh_ligand_2_15.mol2 | 4uxh | 1 | -6.21 | C([C@H]1OCC[C@@H]1O)O | 8 |
| 2yaz_ligand_2_3.mol2 | 2yaz | 1 | -6.17 | C(O)[C@@H]1[C@H](CCO1)O | 8 |
| 1z34_ligand_1_0.mol2 | 1z34 | 1 | -6.09 | C(O)[C@H]1OCC[C@@H]1O | 8 |
| 3ipx_ligand_1_1.mol2 | 3ipx | 1 | -6.07 | C1O[C@@H]([C@H](C1)O)CO | 8 |
| 4knz_ligand_2_5.mol2 | 4knz | 1 | -6.07 | OC[C@@H]1[C@H](CCO1)O | 8 |
| 2qch_ligand_2_3.mol2 | 2qch | 1 | -6.05 | C(O)[C@@H]1[C@H](CCO1)O | 8 |
171 ,
18

