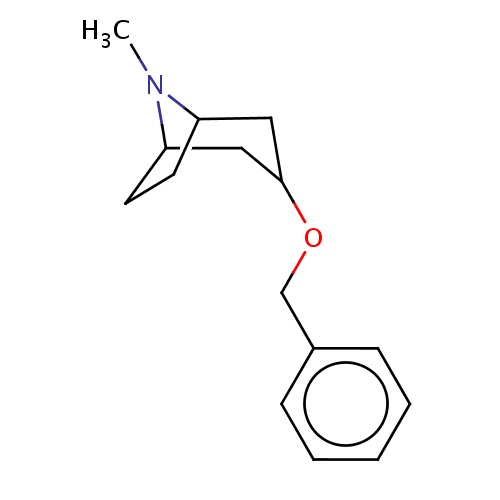
Common name
(1R,5S)-3-benzyloxy-8-methyl-8-azabicyclo[3.2.1]octane
IUPAC name
(1R,5S)-3-benzyloxy-8-methyl-8-azabicyclo[3.2.1]octane
SMILES
c1(ccccc1)COC2CC3N(C(C2)CC3)C
Common name
(1R,5S)-3-benzyloxy-8-methyl-8-azabicyclo[3.2.1]octane
IUPAC name
(1R,5S)-3-benzyloxy-8-methyl-8-azabicyclo[3.2.1]octane
SMILES
c1(ccccc1)COC2CC3N(C(C2)CC3)C
INCHI
InChI=1S/C15H21NO/c1-16-13-7-8-14(16)10-15(9-13)17-11-12-5-3-2-4-6-12/h2-6,13-15H,7-11H2,1H3/t13-,14+,15+
FORMULA
C15H21NO

Common name
(1R,5S)-3-benzyloxy-8-methyl-8-azabicyclo[3.2.1]octane
IUPAC name
(1R,5S)-3-benzyloxy-8-methyl-8-azabicyclo[3.2.1]octane
Molecular weight
231.333
clogP
2.564
clogS
-2.764
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
12.47
Number of Rings
3
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00133 | Benzatropine |
|
Dopamine Uptake Inhibitors; Antiparkinson Agents; Muscarinic Antagonists; Parasympatholytics; Antidyskinetics; Nervous System; Ethers of Tropine or Tropine Derivatives; Anti-Parkinson Drugs; Anticholinergics; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For use as an adjunct in the therapy of all forms of parkinsonism and also for use in the control of extrapyramidal disorders due to neuroleptic drugs. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1i7z_ligand_1_1.mol2 | 1i7z | 0.861111 | -8.63 | C1[C@H](C[C@H]2[N@@H+]([C@@H]1CC2)C)OC(=O)c1ccccc1 | 18 |
| 2y57_ligand_2_0.mol2 | 2y57 | 0.861111 | -8.56 | C[N@@H+]1[C@H]2C[C@@H](C[C@@H]1CC2)OC(=O)c1ccccc1 | 18 |
| 2pgz_ligand_1_1.mol2 | 2pgz | 0.861111 | -8.50 | C1[C@@H]2[N@H+]([C@@H](CC2)C[C@@H]1OC(=O)c1ccccc1)C | 18 |
| 2y58_ligand_2_0.mol2 | 2y58 | 0.861111 | -8.50 | O=C(O[C@H]1C[C@H]2CC[C@@H](C1)[N+]2(C)C)c1ccccc1 | 19 |
| 2y58_ligand_1_0.mol2 | 2y58 | 0.861111 | -8.38 | O=C(O[C@H]1C[C@H]2CC[C@@H](C1)[N@@H+]2C)c1ccccc1 | 18 |
| 2y56_ligand_2_0.mol2 | 2y56 | 0.861111 | -8.10 | C[N@H+]1[C@@H]2C[C@@H](OC(=O)c3ccccc3)C[C@H]1CC2 | 18 |
| 1q72_ligand_1_1.mol2 | 1q72 | 0.861111 | -7.88 | C1[C@@H]2[N@H+]([C@@H](CC2)C[C@@H]1OC(=O)c1ccccc1)C | 18 |
| 2y57_ligand_1_0.mol2 | 2y57 | 0.847222 | -8.48 | [C@@H]1(C[C@H]2CC[C@@H](C1)[NH2+]2)OC(=O)c1ccccc1 | 17 |
| 2y54_ligand.mol2 | 2y54 | 0.847222 | -8.17 | c1ccccc1C(=O)O[C@H]1C[C@H]2CC[C@@H](C1)[NH2+]2 | 18 |
| 2y56_ligand_1_0.mol2 | 2y56 | 0.847222 | -7.99 | O=C(O[C@@H]1C[C@@H]2CC[C@H](C1)[NH2+]2)c1ccccc1 | 17 |
105 ,
11

