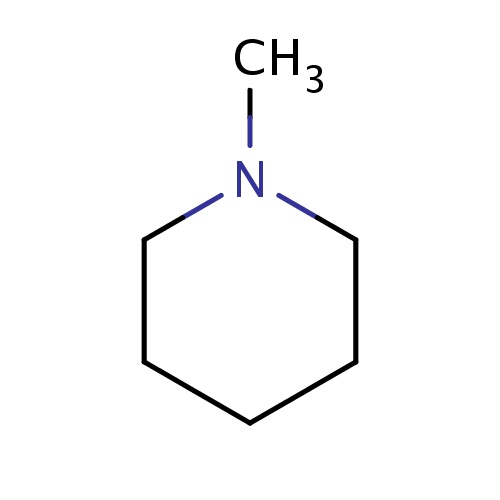
Common name
1-methylpiperidine
IUPAC name
1-methylpiperidine
SMILES
CN1CCCCC1
Common name
1-methylpiperidine
IUPAC name
1-methylpiperidine
SMILES
CN1CCCCC1
INCHI
InChI=1S/C6H13N/c1-7-5-3-2-4-6-7/h2-6H2,1H3
FORMULA
C6H13N

Common name
1-methylpiperidine
IUPAC name
1-methylpiperidine
Molecular weight
99.174
clogP
1.483
clogS
-0.911
Frequency
0.0172
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01108 | Paliperidone |

|
Antipsychotic Agents; Adrenergic alpha-1 Receptor Antagonists; Nervous System; Psycholeptics; Dopamine D2 Receptor Antagonists; Serotonin 5-HT2 Receptor Antagonists; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Alpha2 Agonists; | For the treatment of schizophrenia. |
| FDBD01141 | Pancuronium |

|
Neuromuscular Nondepolarizing Agents; Nicotinic Antagonists; Musculo-Skeletal System; Muscle Relaxants; Muscle Relaxants, Peripherally Acting Agents; | Used as a muscle relaxant during anesthesia and surgical procedures. |
| FDBD01143 | Vecuronium |

|
Neuromuscular Nondepolarizing Agents; Nicotinic Antagonists; Musculo-Skeletal System; Muscle Relaxants; Muscle Relaxants, Peripherally Acting Agents; | Vecuronium is a muscle relaxing agent and is used as an ajunct in general anesthesia. |
| FDBD01270 | Pipotiazine |

|
Antipsychotic Agents; Phenothiazines; Nervous System; Psycholeptics; Phenothiazines With Piperidine Structure; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the maintenance treatment of chronic non-agitated schizophrenic patients. |
| FDBD01342 | Bepotastine |
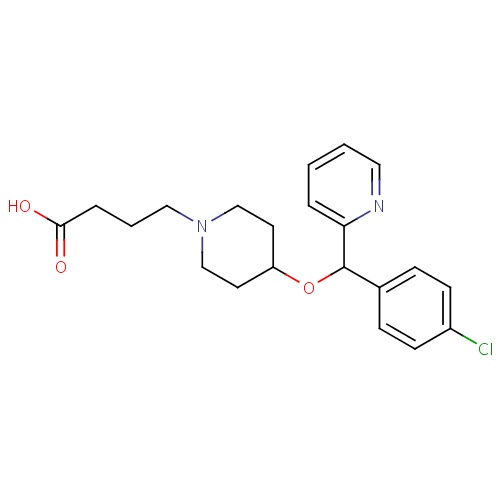
|
Mast Cell Stabilizers; | For the symptomatic treatment of itchy eyes (caused by IgE-induced mast cell degranulation) due to allergic conjunctivitis. |
| FDBD01352 | Iloperidone |
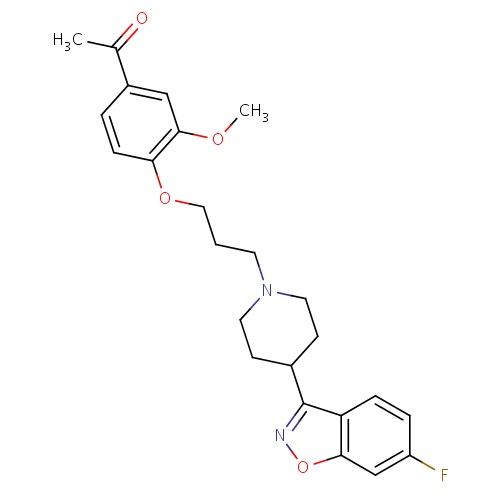
|
Antipsychotic Agents; Adrenergic alpha-1 Receptor Antagonists; Nervous System; Psycholeptics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | Treatment of acute schizophrenia. |
| FDBD01367 | Vandetanib |
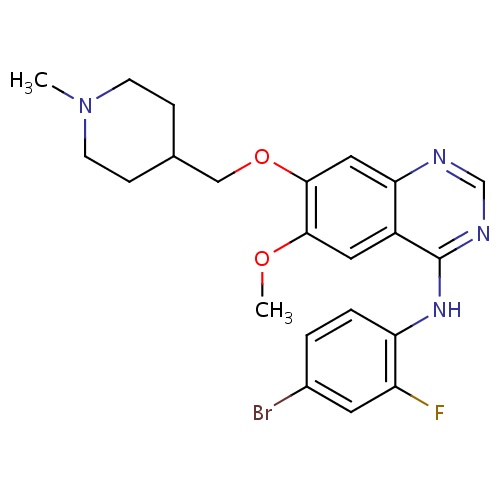
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; CYP3A4 Inhibitors; | Vandetanib is currently approved as an alternative to local therapies for both unresectable and disseminated disease. Because Vandetanib can prolong the Q-T interval, it is contraindicated for use in patients with serious cardiac complications such as congenital long QT syndrome and uncompensated heart failure. |
| FDBD01374 | Sertindole |

|
Antipsychotic Agents; Nervous System; Psycholeptics; Indole Derivatives; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Used in the treatment of schizophrenia. |
| FDBD01418 | Prucalopride |
|
Alimentary Tract and Metabolism; Drugs for Constipation; | Investigated for use/treatment in constipation, ileus, and pediatric indications. |
| FDBD01464 | Ketobemidone |

|
Analgesics; Analgesics, Opioid; Excitatory Amino Acid Antagonists; Nervous System; Opioids; Phenylpiperidine Derivatives; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | For the treatment of all types of severe pain, such as postoperative, cancer, kidney stones and fractures. |
50 ,
6
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2bys_ligand_frag_3.mol2 | 2bys | 1 | -6.50 | C1CCCC[NH+]1C | 7 |
| 2iog_ligand_1_10.mol2 | 2iog | 1 | -6.37 | [NH+]1(CCCCC1)C | 7 |
| 3m8q_ligand_1_4.mol2 | 3m8q | 1 | -6.30 | C[NH+]1CCCCC1 | 7 |
| 1o79_ligand_1_0.mol2 | 1o79 | 1 | -6.29 | C1CC[NH+](CC1)C | 7 |
| 1eve_ligand_1_4.mol2 | 1eve | 1 | -6.25 | C[NH+]1CCCCC1 | 7 |
| 3i6m_ligand_1_4.mol2 | 3i6m | 1 | -6.20 | [NH+]1(CCCCC1)C | 7 |
| 4li8_ligand_1_4.mol2 | 4li8 | 1 | -6.20 | [NH+]1(CCCCC1)C | 7 |
| 2y1w_ligand_1_2.mol2 | 2y1w | 1 | -6.18 | C[NH+]1CCCCC1 | 7 |
123 ,
13

