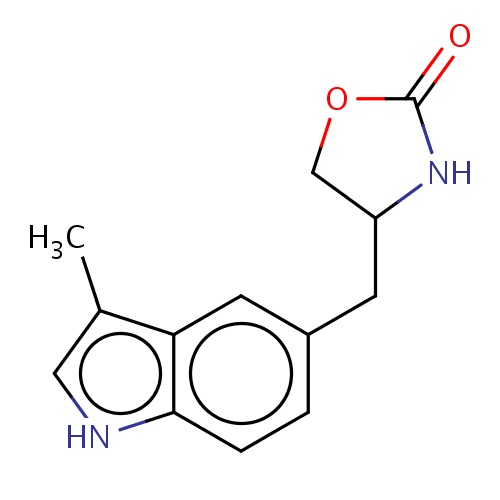
Common name
(4S)-4-[(3-methyl-1H-indol-5-yl)methyl]oxazolidin-2-one
IUPAC name
(4S)-4-[(3-methyl-1H-indol-5-yl)methyl]oxazolidin-2-one
SMILES
[nH]1c2c(c(c1)C)cc(cc2)CC3COC(=O)N3
Common name
(4S)-4-[(3-methyl-1H-indol-5-yl)methyl]oxazolidin-2-one
IUPAC name
(4S)-4-[(3-methyl-1H-indol-5-yl)methyl]oxazolidin-2-one
SMILES
[nH]1c2c(c(c1)C)cc(cc2)CC3COC(=O)N3
INCHI
InChI=1S/C13H14N2O2/c1-8-6-14-12-3-2-9(5-11(8)12)4-10-7-17-13(16)15-10/h2-3,5-6,10,14H,4,7H2,1H3,(H,15,16)/t10-/m0/s1
FORMULA
C13H14N2O2

Common name
(4S)-4-[(3-methyl-1H-indol-5-yl)methyl]oxazolidin-2-one
IUPAC name
(4S)-4-[(3-methyl-1H-indol-5-yl)methyl]oxazolidin-2-one
Molecular weight
230.262
clogP
2.919
clogS
-3.758
Frequency
0.0003
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
54.12
Number of Rings
3
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
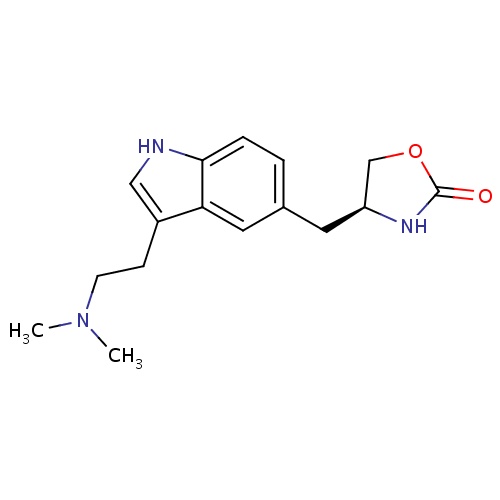
| FDBD00201 | Zolmitriptan |
|
Analgesics; Serotonin Antagonists; Serotonin 5-HT1 Receptor Agonists; Serotonin Receptor Agonists; Anti-migraine Agents; Nervous System; Selective Serotonin (5Ht1) Agonists; Antimigraine Preparations; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; | For the acute treatment of adult migraine with or without auras. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4n4v_ligand_1_1.mol2 | 4n4v | 0.710145 | -7.79 | C1C(OC(=O)N1C1CCCCC1)(C)C | 14 |
| 4oo9_ligand_frag_1.mol2 | 4oo9 | 0.657143 | -7.62 | N1([C@@H]2CCC[C@@H]([C@@H]2CC1)O)C(=O)OC | 14 |
| 1xdg_ligand_4_145.mol2 | 1xdg | 0.633803 | -5.54 | CN1[C@H](C[C@H](OC1=O)CC)C | 11 |
| 1xdg_ligand_3_90.mol2 | 1xdg | 0.633803 | -5.50 | N1[C@H](C[C@H](OC1=O)CC)C | 10 |
| 4lnb_ligand_2_0.mol2 | 4lnb | 0.615385 | -6.44 | O=C(OC(C)(C)C)N1CCC(CC1)C | 14 |
| 2pjt_ligand_2_26.mol2 | 2pjt | 0.615385 | -5.99 | CC1CCN(CC1)C(=O)OC(C)(C)C | 14 |
| 4dfg_ligand_1_10.mol2 | 4dfg | 0.604938 | -6.49 | N(C(=O)OC)[C@@H]1CO[C@@H]2CCC[C@H]12 | 13 |
| 3elm_ligand_1_6.mol2 | 3elm | 0.6 | -6.37 | C(C)(C)OC(=O)N1CCCCC1 | 12 |
| 4lnb_ligand_1_0.mol2 | 4lnb | 0.6 | -6.30 | O=C(OC(C)(C)C)N1CCCCC1 | 13 |
138 ,
14

