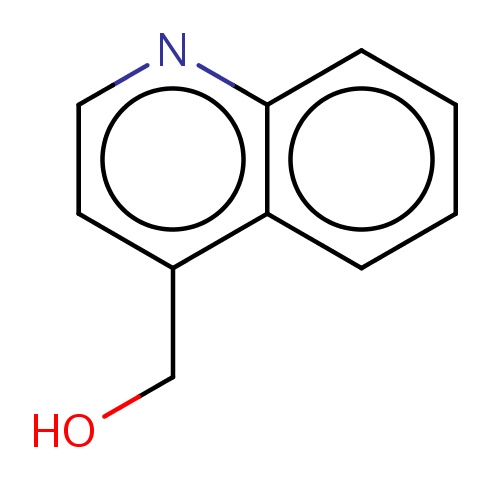
Common name
4-quinolylmethanol
IUPAC name
4-quinolylmethanol
SMILES
C(O)c1c2c(ncc1)cccc2
Common name
4-quinolylmethanol
IUPAC name
4-quinolylmethanol
SMILES
C(O)c1c2c(ncc1)cccc2
INCHI
InChI=1S/C10H9NO/c12-7-8-5-6-11-10-4-2-1-3-9(8)10/h1-6,12H,7H2
FORMULA
C10H9NO

Common name
4-quinolylmethanol
IUPAC name
4-quinolylmethanol
Molecular weight
159.185
clogP
2.312
clogS
-2.610
Frequency
0.0014
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
33.12
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
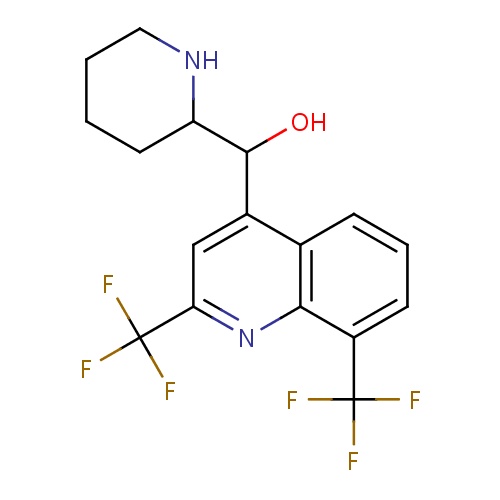
| FDBD00242 | Mefloquine |
|
Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Methanolquinolines; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of mild to moderate acute malaria caused by Mefloquineuine-susceptible strains of . |
| FDBD00341 | Quinine |

|
Antimalarials; Antiprotozoal Agents; Analgesics, Non-Narcotic; Muscle Relaxants, Central; Musculo-Skeletal System; Antiparasitic Products, Insecticides and Repellents; Methanolquinolines; Quinine and Derivatives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of malaria and leg cramps. |
| FDBD00765 | Quinidine |

|
Enzyme Inhibitors; Anti-Arrhythmia Agents; Antimalarials; Muscarinic Antagonists; Voltage-Gated Sodium Channel Blockers; Adrenergic alpha-Antagonists; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ia; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; BSEP/ABCB11 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of ventricular pre-excitation and cardiac dysrhythmias. |
| FDBD01146 | Quinidine barbiturate |

|
; |
4 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3e8r_ligand_2_25.mol2 | 3e8r | 1 | -6.86 | c1cc2c(ccnc2cc1)CO | 12 |
| 4uin_ligand_1_1.mol2 | 4uin | 1 | -6.50 | C(O)c1ccnc2c1cccc2 | 12 |
| 4uil_ligand_1_1.mol2 | 4uil | 1 | -6.40 | c1(ccnc2c1cccc2)CO | 12 |
| 2fv5_ligand_2_0.mol2 | 2fv5 | 0.833333 | -7.75 | OCc1c2ccccc2nc(c1)C | 13 |
| 4nj3_ligand_frag_0.mol2 | 4nj3 | 0.833333 | -7.74 | n1ccc(c2c1cccc2)C(=O)O | 13 |
| 3e8r_ligand_1_6.mol2 | 3e8r | 0.8 | -7.03 | c1cc2c(ccnc2cc1)C | 11 |
| 4dvi_ligand_frag_1.mol2 | 4dvi | 0.8 | -6.85 | n1c2ccccc2c(cc1)C | 11 |
| 4ej8_ligand.mol2 | 4ej8 | 0.78 | -6.32 | O=C(O)c1ccc2cc[nH]c2c1 | 13 |
101 ,
11

