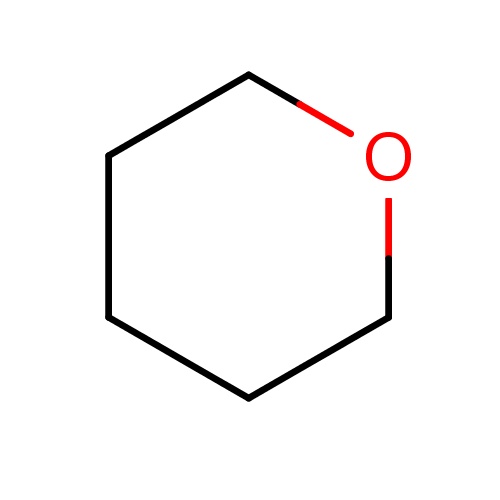
Common name
tetrahydropyran
IUPAC name
tetrahydropyran
SMILES
C1OCCCC1
Common name
tetrahydropyran
IUPAC name
tetrahydropyran
SMILES
C1OCCCC1
INCHI
InChI=1S/C5H10O/c1-2-4-6-5-3-1/h1-5H2
FORMULA
C5H10O

Common name
tetrahydropyran
IUPAC name
tetrahydropyran
Molecular weight
86.132
clogP
2.070
clogS
-0.902
Frequency
0.0017
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
9.23
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00248 | Sucralfate |
|
Anti-Ulcer Agents; Alimentary Tract and Metabolism; Drugs for Peptic Ulcer and Gastro-Oesophageal Reflux Disease (Gord); Drugs for Acid Related Disorders; | For the short-term treatment (up to 8 weeks) of active duodenal ulcer, as well as maintenance therapy for duodenal ulcer patients at reduced dosage (1 gram twice a day) after healing of acute ulcers. Also used for the short-term treatment of gastric ulcer. |
| FDBD00436 | Fondaparinux sodium |

|
Antithrombins; Anticoagulants; | Approved for: (1) prophylaxis of VTE for up to one month post surgery in patients undergoing orthopedic surgery of the lower limbs such as hip fracture, hip replacement and knee surgery; (2) prophylaxis of VTE patients undergoing abdominal surgery who are at high risk of thromboembolic complications (e.g. patients undergoing abdominal cancer surgery); (3) treatment of acute DVT and PE; (4) management of UA and NSTEMI for the prevention of death and subsequent myocardial infarction (MI); and (5) management of STEMI for the prevention of death and myocardial reinfarction in patients who are managed with thrombolytics or who are initially to receive no form of reperfusion therapy. Fondaparinux should not be used as the sole anticoagulant during percutaneous coronary intervention (PCI) due to an increased risk of guiding catheter thrombosis. |
| FDBD00849 | Auranofin |

|
Antirheumatic Agents; Musculo-Skeletal System; Antiinflammatory and Antirheumatic Products; Specific Antirheumatic Agents; Gold Preparations; | Used in the treatment of active, progressive or destructive forms of inflammatory arthritis, such as adult rheumatoid arthritis. |
| FDBD01838 | Venetoclax |

|
; | For the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion, as detected by an FDA approved test, who have received at least one prior therapy. |
| FDBD02508 | tepraloxydim |

|
Herbicide | Herbicide |
5 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 5bve_ligand_frag_5.mol2 | 5bve | 1 | -5.99 | C1CCOCC1 | 6 |
| 5bvd_ligand_frag_4.mol2 | 5bvd | 1 | -5.97 | C1CCOCC1 | 6 |
| 4xj0_ligand_frag_4.mol2 | 4xj0 | 1 | -5.95 | C1CCCOC1 | 6 |
| 2wf4_ligand_frag_6.mol2 | 2wf4 | 1 | -5.82 | C1CCOCC1 | 6 |
| 4o6e_ligand_frag_3.mol2 | 4o6e | 1 | -5.82 | C1CCCOC1 | 6 |
| 4phw_ligand_frag_4.mol2 | 4phw | 1 | -5.81 | C1CCOCC1 | 6 |
| 3mj1_ligand_frag_4.mol2 | 3mj1 | 1 | -5.80 | C1CCOCC1 | 6 |
158 ,
16

