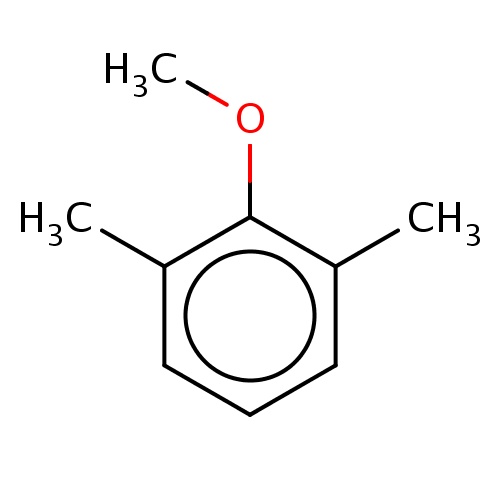
Common name
2-methoxy-1,3-dimethyl-benzene
IUPAC name
2-methoxy-1,3-dimethyl-benzene
SMILES
c1(c(cccc1C)C)OC
Common name
2-methoxy-1,3-dimethyl-benzene
IUPAC name
2-methoxy-1,3-dimethyl-benzene
SMILES
c1(c(cccc1C)C)OC
INCHI
InChI=1S/C9H12O/c1-7-5-4-6-8(2)9(7)10-3/h4-6H,1-3H3
FORMULA
C9H12O

Common name
2-methoxy-1,3-dimethyl-benzene
IUPAC name
2-methoxy-1,3-dimethyl-benzene
Molecular weight
136.191
clogP
2.762
clogS
-2.814
Frequency
0.0007
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
9.23
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00261 | Mexiletine |

|
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ib; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of ventricular tachycardia and symptomatic premature ventricular beats, and prevention of ventricular fibrillation. |
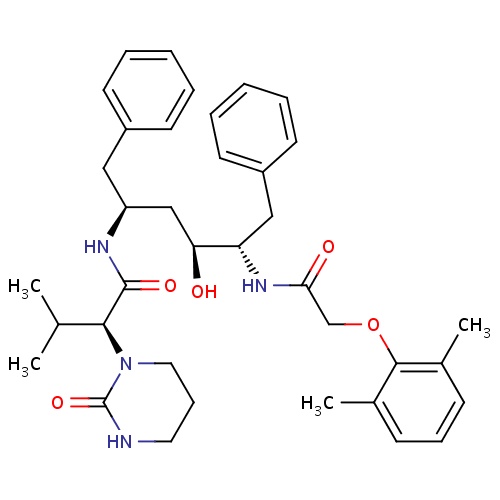
| FDBD01251 | Lopinavir |
|
Anti-HIV Agents; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with other antiretroviral agents for the treatment of HIV-infection. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3fnu_ligand_2_9.mol2 | 3fnu | 1 | -6.91 | COc1c(cccc1C)C | 10 |
| 2o4s_ligand_2_104.mol2 | 2o4s | 1 | -6.76 | COc1c(cccc1C)C | 10 |
| 4b1d_ligand_1_1.mol2 | 4b1d | 1 | -6.71 | O(C)c1c(C)cccc1C | 10 |
| 2q5k_ligand_2_104.mol2 | 2q5k | 1 | -6.70 | c1(c(cccc1C)C)OC | 10 |
| 1idb_ligand_2_12.mol2 | 1idb | 1 | -6.47 | COc1c(cccc1C)C | 10 |
| 1mui_ligand_2_104.mol2 | 1mui | 1 | -6.45 | c1(c(cccc1C)C)OC | 10 |
| 2rkg_ligand_2_104.mol2 | 2rkg | 1 | -6.42 | c1(c(cccc1C)C)OC | 10 |
| 2qhc_ligand_2_104.mol2 | 2qhc | 1 | -6.40 | c1(c(cccc1C)C)OC | 10 |
166 ,
17

