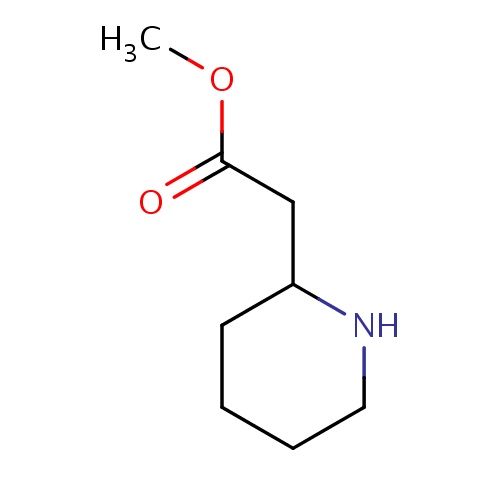
Common name
methyl 2-[(2S)-2-piperidyl]acetate
IUPAC name
methyl 2-[(2S)-2-piperidyl]acetate
SMILES
C(C(=O)OC)C1NCCCC1
Common name
methyl 2-[(2S)-2-piperidyl]acetate
IUPAC name
methyl 2-[(2S)-2-piperidyl]acetate
SMILES
C(C(=O)OC)C1NCCCC1
INCHI
InChI=1S/C8H15NO2/c1-11-8(10)6-7-4-2-3-5-9-7/h7,9H,2-6H2,1H3/t7-/m0/s1
FORMULA
C8H15NO2

Common name
methyl 2-[(2S)-2-piperidyl]acetate
IUPAC name
methyl 2-[(2S)-2-piperidyl]acetate
Molecular weight
157.210
clogP
1.220
clogS
-1.557
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
38.33
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00299 | Methylphenidate |
|
Central Nervous System Stimulants; Dopamine Uptake Inhibitors; Nervous System; Psychoanaleptics; Centrally Acting Sympathomimetics; Psychostimulants, Agents Used for Adhd and Nootropics; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For use as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate-to-severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2v11_ligand_2_12.mol2 | 2v11 | 0.839286 | -6.87 | [N@@H+]1(C[C@H]([C@H]2[C@H]1CCCC2)C(=O)OC)C | 14 |
| 2v11_ligand_1_2.mol2 | 2v11 | 0.839286 | -6.75 | [NH2+]1C[C@H]([C@H]2[C@H]1CCCC2)C(=O)OC | 13 |
| 4rvr_ligand_2_4.mol2 | 4rvr | 0.781818 | -6.73 | O([C@@H]1C[C@H]2[N@@H+](CC1)[C@H](C(=O)C)CC2)C | 14 |
| 4rvr_ligand_3_6.mol2 | 4rvr | 0.767857 | -7.52 | O([C@@H]1C[C@H]2[N@@H+](CC1)[C@H](C(=O)C)C[C@H]2C1CCCCC1)C | 20 |
| 3bgz_ligand_1_1.mol2 | 3bgz | 0.764706 | -8.50 | C1[C@@H]([NH2+][C@H]2[C@H](CCC[C@H]12)C(=O)O)C1CCCCC1 | 18 |
| 3bgz_ligand_1_0.mol2 | 3bgz | 0.764706 | -8.46 | [C@@H]1(C[NH2+][C@H]2[C@H](CCC[C@H]12)C(=O)O)C1CCCCC1 | 18 |
| 3fr2_ligand_1_0.mol2 | 3fr2 | 0.764706 | -8.10 | C[N@H+]1[C@H]2[C@@H]([C@H]3CCC[C@H](C(=O)O)[C@@H]13)CCCC2 | 17 |
| 3fr4_ligand_1_2.mol2 | 3fr4 | 0.764706 | -8.02 | [N@@H+]1([C@@H]2CCCC[C@H]2[C@H]2[C@H]1[C@@H](CCC2)C(=O)O)C | 17 |
101 ,
11

