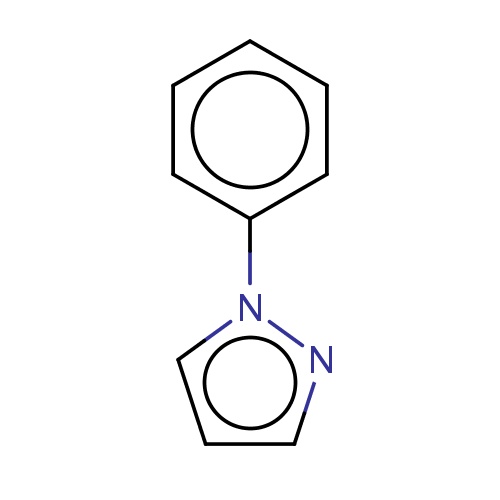
Common name
1-phenylpyrazole
IUPAC name
1-phenylpyrazole
SMILES
n1(nccc1)c2ccccc2
Common name
1-phenylpyrazole
IUPAC name
1-phenylpyrazole
SMILES
n1(nccc1)c2ccccc2
INCHI
InChI=1S/C9H8N2/c1-2-5-9(6-3-1)11-8-4-7-10-11/h1-8H
FORMULA
C9H8N2

Common name
1-phenylpyrazole
IUPAC name
1-phenylpyrazole
Molecular weight
145.181
clogP
0.457
clogS
-1.696
Frequency
0.0007
HBond Acceptor
0
HBond Donor
1
Total PolarSurface Area
19.67
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00355 | Celecoxib |

|
Antineoplastic Agents; Cyclooxygenase 2 Inhibitors; Antineoplastic and Immunomodulating Agents; Musculo-Skeletal System; Antiinflammatory and Antirheumatic Products, Non-Steroids; Antiinflammatory and Antirheumatic Products; Coxibs; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For relief and management of osteoarthritis (OA), rheumatoid arthritis (RA), juvenile rheumatoid arthritis (JRA), ankylosing spondylitis, acute pain, primary dysmenorrhea and oral adjunct to usual care for patients with familial adenomatous polyposis. |
| FDBD01462 | Sulfaphenazole |
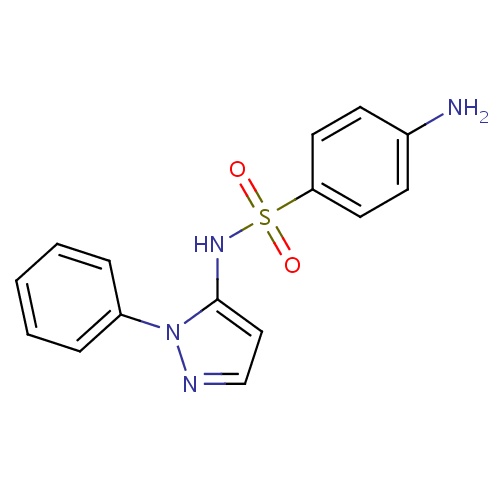
|
Anti-Bacterial Agents; Anti-Infective Agents; Sulfonamides; Ophthalmologicals; Sensory Organs; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Antiinfectives; Sulfonamides and Trimethoprim; Long-Acting Sulfonamides; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the treatment bacterial infections. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4r5t_ligand_1_4.mol2 | 4r5t | 1 | -7.13 | [n+]1([nH]ccc1)c1ccccc1 | 11 |
| 4r5x_ligand_1_1.mol2 | 4r5x | 1 | -7.11 | c1c[nH][n+](c1)c1ccccc1 | 11 |
| 4k5o_ligand_1_2.mol2 | 4k5o | 1 | -7.07 | [n+]1([nH]ccc1)c1ccccc1 | 11 |
| 4r5v_ligand_1_4.mol2 | 4r5v | 1 | -7.04 | [n+]1([nH]ccc1)c1ccccc1 | 11 |
| 3n4l_ligand_1_6.mol2 | 3n4l | 1 | -6.75 | [n+]1([nH]ccc1)c1ccccc1 | 11 |
| 4k3n_ligand_1_2.mol2 | 4k3n | 1 | -6.62 | c1ccc(cc1)[n+]1[nH]ccc1 | 11 |
101 ,
11

