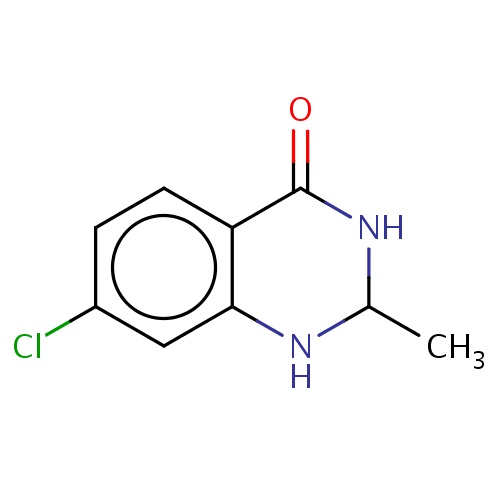
Common name
(2S)-7-chloro-2-methyl-2,3-dihydro-1H-quinazolin-4-one
IUPAC name
(2S)-7-chloro-2-methyl-2,3-dihydro-1H-quinazolin-4-one
SMILES
Clc1cc2c(cc1)C(=O)NC(N2)C
Common name
(2S)-7-chloro-2-methyl-2,3-dihydro-1H-quinazolin-4-one
IUPAC name
(2S)-7-chloro-2-methyl-2,3-dihydro-1H-quinazolin-4-one
SMILES
Clc1cc2c(cc1)C(=O)NC(N2)C
INCHI
InChI=1S/C9H9ClN2O/c1-5-11-8-4-6(10)2-3-7(8)9(13)12-5/h2-5,11H,1H3,(H,12,13)/t5-/m0/s1
FORMULA
C9H9ClN2O

Common name
(2S)-7-chloro-2-methyl-2,3-dihydro-1H-quinazolin-4-one
IUPAC name
(2S)-7-chloro-2-methyl-2,3-dihydro-1H-quinazolin-4-one
Molecular weight
196.634
clogP
1.842
clogS
-3.263
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
41.13
Number of Rings
2
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00393 | Metolazone |
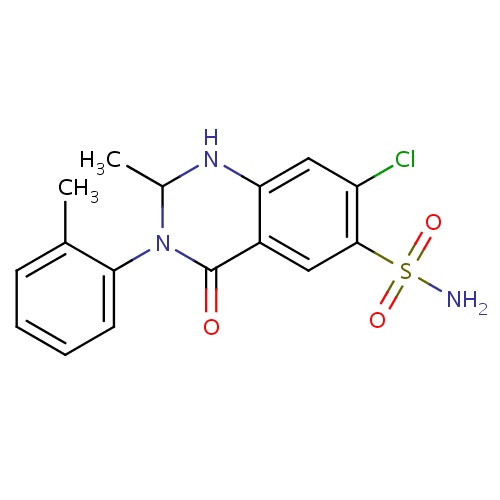
|
Antihypertensive Agents; Diuretics; Sodium Chloride Symporter Inhibitors; Cardiovascular System; Sulfonamides, Plain; Low-Ceiling Diuretics, Excl. Thiazides; Low-Ceiling Diuretics and Potassium-Sparing Agents; | For the treatment of hypertension, alone or in combination with other antihypertensive drugs of a different class. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 5akw_ligand.mol2 | 5akw | 0.88785 | -9.40 | Clc1ccc(cc1)[C@@H]1NC(=O)c2ccccc2N1 | 19 |
| 5aku_ligand_1_1.mol2 | 5aku | 0.803738 | -9.21 | c1ccc(cc1)[C@@H]1NC(=O)c2c(N1)cccc2 | 17 |
| 5aku_ligand.mol2 | 5aku | 0.785714 | -10.29 | CC(C)(C)c1ccc(cc1)[C@@H]1NC(=O)c2ccccc2N1 | 22 |
| 5aku_ligand_frag_0.mol2 | 5aku | 0.752475 | -7.63 | N1CNc2c(C1=O)cccc2 | 11 |
| 5akw_ligand_frag_1.mol2 | 5akw | 0.752475 | -7.57 | O=C1NCNc2ccccc12 | 11 |
| 5al3_ligand.mol2 | 5al3 | 0.605263 | -9.48 | Clc1cc(Cl)ccc1[C@@H]1NC(=O)c2cccnc2N1C | 21 |
| 4hbw_ligand_frag_2.mol2 | 4hbw | 0.594595 | -6.91 | c1cc2CN(C(=O)Nc2cc1)C | 12 |
| 4hbx_ligand_frag_0.mol2 | 4hbx | 0.594595 | -6.84 | c1ccc2NC(=O)N(Cc2c1)C | 12 |
| 4hby_ligand_frag_0.mol2 | 4hby | 0.594595 | -6.78 | c1ccc2CN(C(=O)Nc2c1)C | 12 |
| 5al2_ligand_1_1.mol2 | 5al2 | 0.584507 | -8.80 | c1ccc(cc1)[C@@H]1NC(=O)c2cccnc2N1 | 17 |
105 ,
11

