
Common name
quinazolin-7-ol
IUPAC name
quinazolin-7-ol
SMILES
n1c2c(ccc(c2)O)cnc1
Common name
quinazolin-7-ol
IUPAC name
quinazolin-7-ol
SMILES
n1c2c(ccc(c2)O)cnc1
INCHI
InChI=1S/C8H6N2O/c11-7-2-1-6-4-9-5-10-8(6)3-7/h1-5,11H
FORMULA
C8H6N2O

Common name
quinazolin-7-ol
IUPAC name
quinazolin-7-ol
Molecular weight
146.146
clogP
1.496
clogS
-1.974
Frequency
0.0010
HBond Acceptor
3
HBond Donor
1
Total Polar
Surface Area
46.01
Number of Rings
2
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00399 | Erlotinib |
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Also for use, in combination with gemcitabine, as the first-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer. |
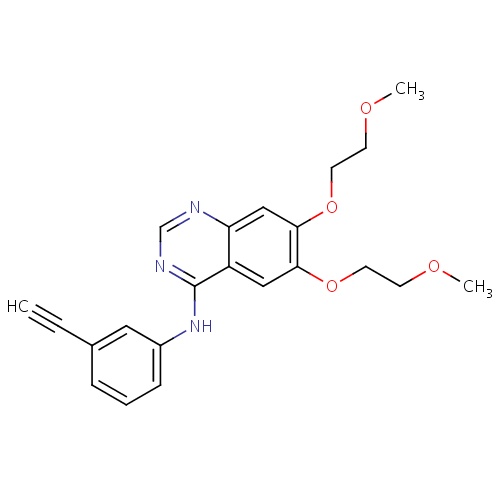
| FDBD01367 | Vandetanib |
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; CYP3A4 Inhibitors; | Vandetanib is currently approved as an alternative to local therapies for both unresectable and disseminated disease. Because Vandetanib can prolong the Q-T interval, it is contraindicated for use in patients with serious cardiac complications such as congenital long QT syndrome and uncompensated heart failure. |
| FDBD01575 | Afatinib |

|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; | Afatinib is a kinase inhibitor indicated for the first-line treatment of patient with metastatic non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4c2v_ligand_1_5.mol2 | 4c2v | 1 | -6.91 | Oc1cc2ncncc2cc1 | 11 |
| 2vrx_ligand_1_4.mol2 | 2vrx | 1 | -6.87 | Oc1cc2c(cc1)cncn2 | 11 |
| 2ivu_ligand_1_2.mol2 | 2ivu | 1 | -6.84 | Oc1cc2c(cc1)cncn2 | 11 |
| 2h8h_ligand_1_3.mol2 | 2h8h | 1 | -6.82 | Oc1ccc2cncnc2c1 | 11 |
| 2h8h_ligand_1_4.mol2 | 2h8h | 1 | -6.82 | c1cc(c2cncnc2c1)O | 11 |
| 2vwu_ligand_1_3.mol2 | 2vwu | 1 | -6.80 | c1cc2cncnc2cc1O | 11 |
| 2c6e_ligand_1_4.mol2 | 2c6e | 1 | -6.68 | Oc1ccc2c(c1)ncnc2 | 11 |
| 3mo5_ligand_1_2.mol2 | 3mo5 | 1 | -6.60 | n1cnc2c(c1)ccc(c2)O | 11 |
| 3mo2_ligand_1_2.mol2 | 3mo2 | 1 | -6.58 | Oc1ccc2c(ncnc2)c1 | 11 |
159 ,
16

