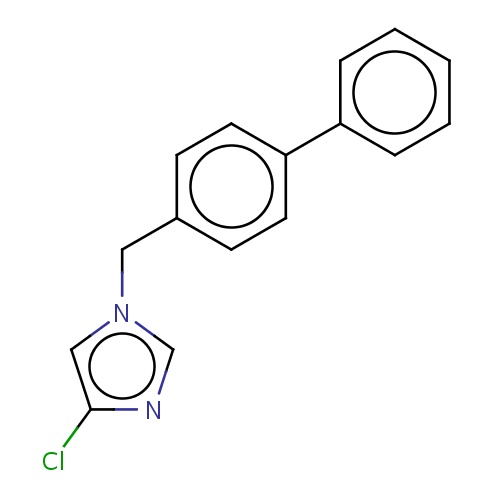
Common name
4-chloro-1-[(4-phenylphenyl)methyl]imidazole
IUPAC name
4-chloro-1-[(4-phenylphenyl)methyl]imidazole
SMILES
C(n1cnc(c1)Cl)c2ccc(cc2)c3ccccc3
Common name
4-chloro-1-[(4-phenylphenyl)methyl]imidazole
IUPAC name
4-chloro-1-[(4-phenylphenyl)methyl]imidazole
SMILES
C(n1cnc(c1)Cl)c2ccc(cc2)c3ccccc3
INCHI
InChI=1S/C16H13ClN2/c17-16-11-19(12-18-16)10-13-6-8-15(9-7-13)14-4-2-1-3-5-14/h1-9,11-12H,10H2
FORMULA
C16H13ClN2

Common name
4-chloro-1-[(4-phenylphenyl)methyl]imidazole
IUPAC name
4-chloro-1-[(4-phenylphenyl)methyl]imidazole
Molecular weight
269.749
clogP
2.881
clogS
-4.804
Frequency
0.0003
HBond Acceptor
0
HBond Donor
1
Total PolarSurface Area
19.67
Number of Rings
3
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
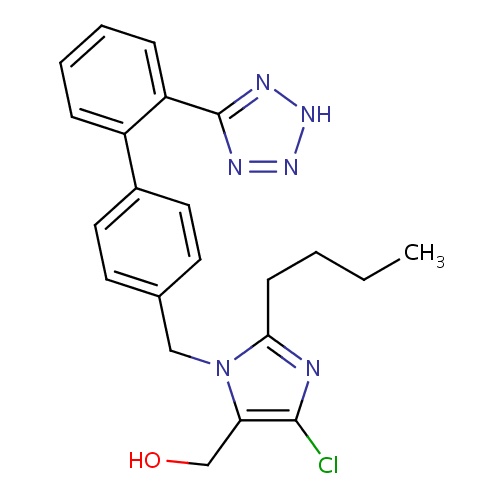
| FDBD00543 | Losartan |
|
Antihypertensive Agents; Anti-Arrhythmia Agents; Angiotensin II Type 1 Receptor Blockers; Angiotensin Receptor Antagonists; Cardiovascular System; Angiotensin II Antagonists, Plain; Agents Acting on the Renin-Angiotensin System; Angiotensin II Antagonists and Diuretics; Angiotensin II Antagonists and Calcium Channel Blockers; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; Angiotensin II Receptor Antagonists; Combined Inhibitors of CYP3A4 and P-glycoprotein; | May be used as a first line agent to treat uncomplicated hypertension, isolated systolic hypertension and left ventricular hypertrophy. May be used as a first line agent to delay progression of diabetic nephropathy. Losartan may be also used as a second line agent in the treatment of congestive heart failure, systolic dysfunction, myocardial infarction and coronary artery disease in those intolerant of ACE inhibitors. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2afx_ligand.mol2 | 2afx | 0.676471 | -6.89 | c1cncn1Cc1ccccc1 | 13 |
| 4d8n_ligand_2_0.mol2 | 4d8n | 0.676471 | -5.94 | [n+]1(cc[nH]c1)Cc1ccccc1 | 12 |
| 1s64_ligand_2_9.mol2 | 1s64 | 0.626667 | -6.48 | c1[n+](c[nH]c1)Cc1ccc(cc1)C#N | 14 |
| 1s63_ligand_2_9.mol2 | 1s63 | 0.626667 | -6.03 | c1[n+](c[nH]c1)Cc1ccc(cc1)C#N | 14 |
| 1n9a_ligand_2_7.mol2 | 1n9a | 0.626667 | -5.71 | c1c(ccc(c1)C#N)C[n+]1c[nH]cc1 | 14 |
| 4d8n_ligand_3_0.mol2 | 4d8n | 0.597403 | -6.11 | [n+]1(cc[nH]c1C)Cc1ccccc1 | 13 |
| 2w71_ligand_2_0.mol2 | 2w71 | 0.586207 | -7.17 | C(c1c(Cl)cccc1Cl)[n+]1cc[nH]c1C | 15 |
102 ,
11

