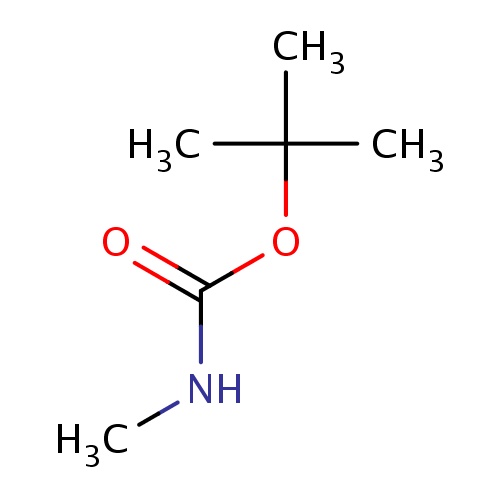
Common name
tert-butyl N-methylcarbamate
IUPAC name
tert-butyl N-methylcarbamate
SMILES
O(C(=O)NC)C(C)(C)C
Common name
tert-butyl N-methylcarbamate
IUPAC name
tert-butyl N-methylcarbamate
SMILES
O(C(=O)NC)C(C)(C)C
INCHI
InChI=1S/C6H13NO2/c1-6(2,3)9-5(8)7-4/h1-4H3,(H,7,8)
FORMULA
C6H13NO2

Common name
tert-butyl N-methylcarbamate
IUPAC name
tert-butyl N-methylcarbamate
Molecular weight
131.173
clogP
0.081
clogS
-1.182
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
38.33
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
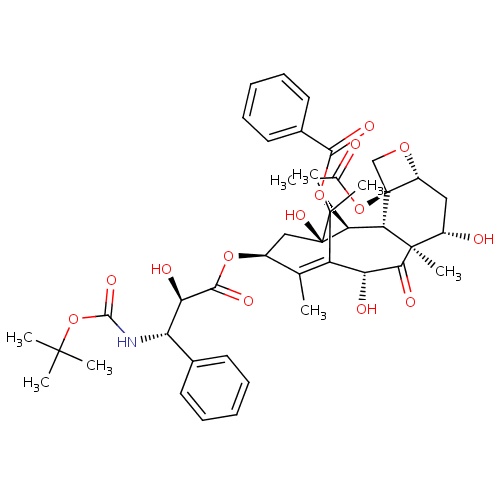
| FDBD01092 | Docetaxel |
|
Antineoplastic Agents; Immunosuppressive Agents; Tubulin Modulators; Antineoplastic and Immunomodulating Agents; Taxanes; CYP3A4 Inhibitors; | For the treatment of patients with locally advanced or metastatic breast cancer after failure of prior chemotherapy. Also used as a single agent in the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of prior platinum-based chemotherapy. It is also used in combination with prednisone, in the treatment of patients with androgen independent (hormone refractory) metastatic prostate cancer. Furthermore, docetaxel has uses in the treatment of gastric adenocarinoma and head and neck cancer. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1zsr_ligand_2_0.mol2 | 1zsr | 1 | -6.54 | CNC(=O)OC(C)(C)C | 9 |
| 1zsf_ligand_2_0.mol2 | 1zsf | 1 | -6.53 | CNC(=O)OC(C)(C)C | 9 |
| 1m0b_ligand_2_0.mol2 | 1m0b | 1 | -6.51 | O=C(OC(C)(C)C)NC | 9 |
| 1lzq_ligand_2_0.mol2 | 1lzq | 1 | -6.50 | O=C(OC(C)(C)C)NC | 9 |
| 1iiq_ligand_2_0.mol2 | 1iiq | 1 | -6.47 | O=C(OC(C)(C)C)NC | 9 |
| 1b6m_ligand_2_0.mol2 | 1b6m | 1 | -6.45 | CNC(=O)OC(C)(C)C | 9 |
| 1izh_ligand_2_168.mol2 | 1izh | 1 | -6.45 | N(C(=O)OC(C)(C)C)C | 9 |
| 1izi_ligand_2_168.mol2 | 1izi | 1 | -6.44 | CNC(=O)OC(C)(C)C | 9 |
| 4zls_ligand_2_71.mol2 | 4zls | 1 | -6.43 | C(C)(C)(C)OC(=O)NC | 9 |
101 ,
11

