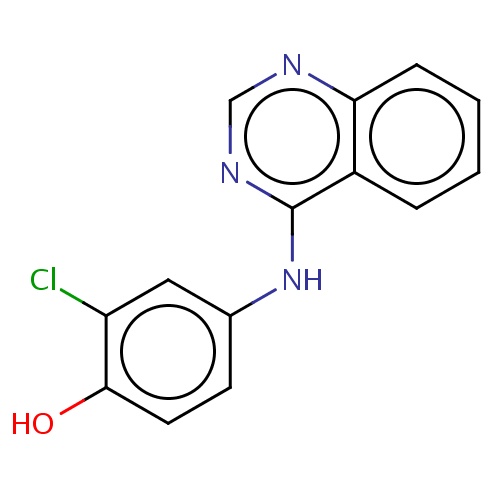
Common name
2-chloro-4-(quinazolin-4-ylamino)phenol
IUPAC name
2-chloro-4-(quinazolin-4-ylamino)phenol
SMILES
Clc1cc(ccc1O)Nc2c3c(ncn2)cccc3
Common name
2-chloro-4-(quinazolin-4-ylamino)phenol
IUPAC name
2-chloro-4-(quinazolin-4-ylamino)phenol
SMILES
Clc1cc(ccc1O)Nc2c3c(ncn2)cccc3
INCHI
InChI=1S/C14H10ClN3O/c15-11-7-9(5-6-13(11)19)18-14-10-3-1-2-4-12(10)16-8-17-14/h1-8,19H,(H,16,17,18)
FORMULA
C14H10ClN3O

Common name
2-chloro-4-(quinazolin-4-ylamino)phenol
IUPAC name
2-chloro-4-(quinazolin-4-ylamino)phenol
Molecular weight
271.702
clogP
2.790
clogS
-4.727
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
58.04
Number of Rings
3
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01101 | Lapatinib |
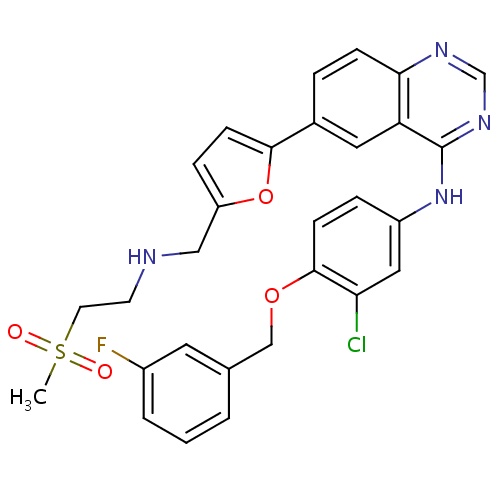
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress the human epidermal receptor type 2 (HER2) protein and who have received prior therapy including an anthracycline, a taxane, and trastuzuma. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1xkk_ligand_3_110.mol2 | 1xkk | 1 | -8.68 | c1ccc2ncnc(c2c1)Nc1ccc(c(Cl)c1)O | 19 |
| 3bbt_ligand_3_0.mol2 | 3bbt | 1 | -8.30 | N(c1ncnc2ccccc12)c1ccc(O)c(Cl)c1 | 19 |
| 1kz8_ligand_3_36.mol2 | 1kz8 | 0.857143 | -6.46 | c1cc(ccc1)Nc1ncnc2c1cc(cc2)O | 18 |
| 2vrx_ligand_3_110.mol2 | 2vrx | 0.785714 | -8.19 | c1(ccccc1)Nc1c2c(ccc(OC)c2)ncn1 | 19 |
| 1di9_ligand_3_3.mol2 | 1di9 | 0.785714 | -7.70 | O(C)c1cc2c(ncnc2Nc2ccccc2)cc1 | 19 |
| 4knx_ligand_3_9.mol2 | 4knx | 0.785714 | -7.54 | c1(cc2c(cc1)ncnc2Nc1ccccc1)OC | 19 |
| 4btk_ligand_2_2.mol2 | 4btk | 0.756098 | -8.62 | N(c1ncnc2c1cccc2)c1cc(ccc1)O | 18 |
| 1di8_ligand_2_0.mol2 | 1di8 | 0.756098 | -8.61 | n1c(c2c(nc1)cccc2)Nc1cccc(c1)O | 18 |
| 2vrx_ligand_3_104.mol2 | 2vrx | 0.756098 | -8.26 | c1(ccccc1)Nc1c2c(cc(O)cc2)ncn1 | 18 |
| 4knx_ligand_3_19.mol2 | 4knx | 0.756098 | -7.44 | c1cc2c(cc1O)ncnc2Nc1ccccc1 | 18 |
101 ,
11

