
Common name
1-[(2S)-2-(1H-imidazol-2-yl)pyrrolidin-1-yl]ethanone
IUPAC name
1-[(2S)-2-(1H-imidazol-2-yl)pyrrolidin-1-yl]ethanone
SMILES
CC(=O)N1C(CCC1)c2[nH]ccn2
Common name
1-[(2S)-2-(1H-imidazol-2-yl)pyrrolidin-1-yl]ethanone
IUPAC name
1-[(2S)-2-(1H-imidazol-2-yl)pyrrolidin-1-yl]ethanone
SMILES
CC(=O)N1C(CCC1)c2[nH]ccn2
INCHI
InChI=1S/C9H13N3O/c1-7(13)12-6-2-3-8(12)9-10-4-5-11-9/h4-5,8H,2-3,6H2,1H3,(H,10,11)/t8-/m0/s1
FORMULA
C9H13N3O

Common name
1-[(2S)-2-(1H-imidazol-2-yl)pyrrolidin-1-yl]ethanone
IUPAC name
1-[(2S)-2-(1H-imidazol-2-yl)pyrrolidin-1-yl]ethanone
Molecular weight
179.219
clogP
1.252
clogS
-1.482
Frequency
0.0007
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
48.99
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01682 | Daclatasvir |

|
Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Daklinza is used to treat patients who have chronic hepatitis C virus (HCV) genotype 3 infection. Daklinza is typically taken in conjunction with sofosbuvir. (2). |
| FDBD01836 | Elbasvir |
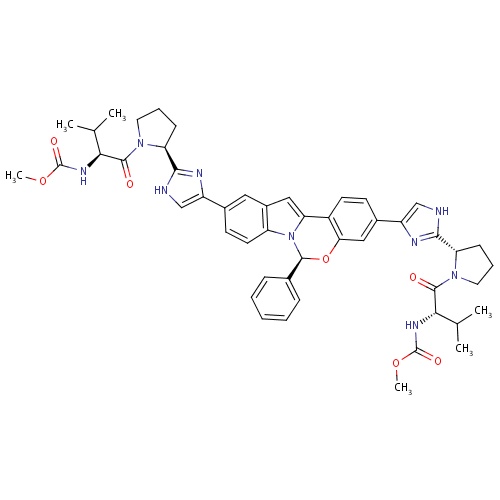
|
; |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1tcw_ligand_4_709.mol2 | 1tcw | 0.803279 | -6.93 | [C@@H](NC(=O)C)(c1[nH+]cc[nH]1)C(C)C | 13 |
| 1bdr_ligand_4_713.mol2 | 1bdr | 0.803279 | -6.85 | c1([nH+]cc[nH]1)[C@H](C(C)C)NC(=O)C | 13 |
| 1bdl_ligand_4_713.mol2 | 1bdl | 0.803279 | -6.80 | CC(=O)N[C@H](c1[nH+]cc[nH]1)C(C)C | 13 |
| 1sbg_ligand_4_709.mol2 | 1sbg | 0.803279 | -6.78 | CC(=O)N[C@H](c1[nH+]cc[nH]1)C(C)C | 13 |
| 1tcx_ligand_4_709.mol2 | 1tcx | 0.803279 | -6.75 | C(=O)(N[C@@H](C(C)C)c1[nH+]cc[nH]1)C | 13 |
| 1bdq_ligand_4_709.mol2 | 1bdq | 0.803279 | -6.71 | CC(=O)N[C@@H](C(C)C)c1[nH+]cc[nH]1 | 13 |
| 4ty6_ligand_4_15.mol2 | 4ty6 | 0.753623 | -7.04 | [C@@H](C)(NC(=O)C1CCCCC1)c1[nH+]cc[nH]1 | 16 |
| 1tcw_ligand_3_285.mol2 | 1tcw | 0.721311 | -6.73 | [C@@H](NC=O)(c1[nH+]cc[nH]1)C(C)C | 12 |
| 1bdl_ligand_3_285.mol2 | 1bdl | 0.721311 | -6.60 | [C@@H](NC=O)(c1[nH+]cc[nH]1)C(C)C | 12 |
| 1bdr_ligand_3_285.mol2 | 1bdr | 0.721311 | -6.57 | c1([nH+]cc[nH]1)[C@H](C(C)C)NC=O | 12 |
102 ,
11

