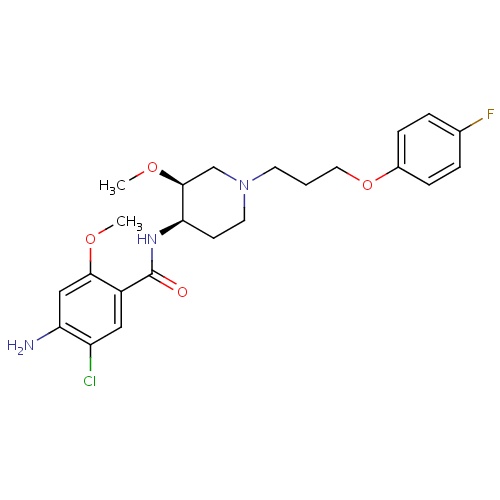
IUPAC name
4-amino-5-chloro-N-[(3S,4R)-1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-yl]-2-methoxybenzamide
SMILES
CO[C@H]1CN(CCCOC2=CC=C(F)C=C2)CC[C@H]1NC(=O)C1=CC(Cl)=C(N)C=C1OC
Compound class
Gastrointestinal Agents; Anti-Ulcer Agents; Serotonin Receptor Agonists; Prokinetic Agents; Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; Propulsives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors;
Therapeutic area
For the symptomatic treatment of adult patients with nocturnal heartburn due to gastroesophageal reflux disease.
Common name
Cisapride
IUPAC name
4-amino-5-chloro-N-[(3S,4R)-1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-yl]-2-methoxybenzamide
SMILES
CO[C@H]1CN(CCCOC2=CC=C(F)C=C2)CC[C@H]1NC(=O)C1=CC(Cl)=C(N)C=C1OC
INCHI
InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29)/t20-,22+/m1/s1
FORMULA
C23H29ClFN3O4

Common name
Cisapride
IUPAC name
4-amino-5-chloro-N-[(3S,4R)-1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-yl]-2-methoxybenzamide
Molecular weight
465.945
clogP
3.526
clogS
-5.876
HBond Acceptor
5
HBond Donor
3
Total Polar Surface Area
86.05
Number of Rings
3
Rotatable Bond
9
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
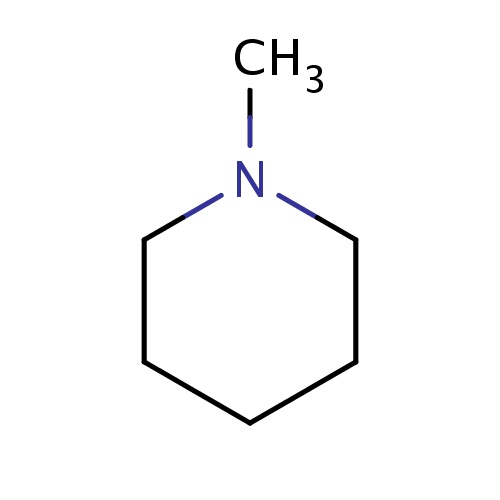
| FDBF00663 | 1-methylpiperidine |
|
CN1CCCCC1 | 0.0172 |
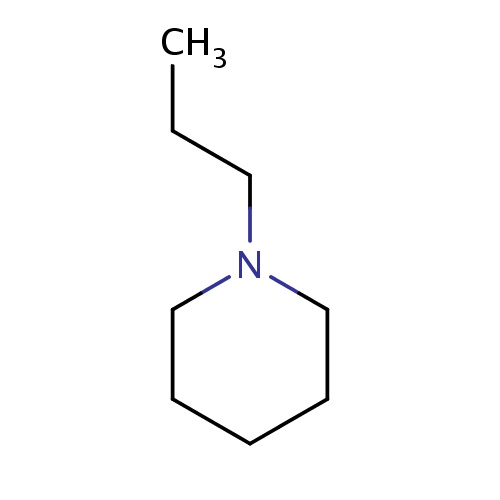
| FDBF00666 | 1-propylpiperidine |
|
C(C)CN1CCCCC1 | 0.0041 |
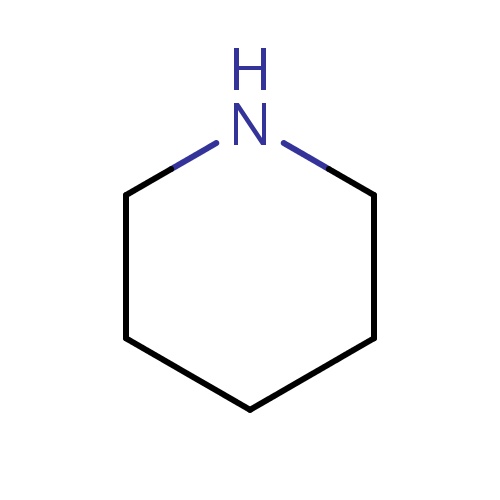
| FDBF00670 | piperidine |
|
N1CCCCC1 | 0.0199 |
| FDBF01358 | N-(4-piperidyl)formamide |

|
O=CNC1CCNCC1 | 0.0027 |
| FDBF01359 | N-(1-methyl-4-piperidyl)formamide |

|
O=CNC1CCN(CC1)C | 0.0027 |
| FDBF01360 | (3R)-3-methoxy-1-methyl-piperidine |

|
O(C)C1CN(CCC1)C | 0.0003 |
| FDBF01368 | N-(1-propyl-4-piperidyl)formamide |

|
O=CNC1CCN(CC1)CCC | 0.0010 |
| FDBF01370 | N-[(3S,4R)-1-ethyl-3-methoxy-4-piperidyl]formamide |

|
O(C)C1CN(CCC1NC=O)CC | 0.0003 |
| FDBF01373 | 3-(1-piperidyl)propan-1-ol |
|
OCCCN1CCCCC1 | 0.0010 |

