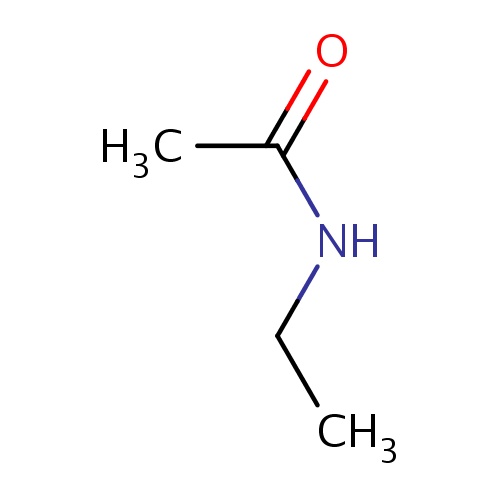
Common name
N-ethylacetamide
IUPAC name
N-ethylacetamide
SMILES
C(=O)(NCC)C
Common name
N-ethylacetamide
IUPAC name
N-ethylacetamide
SMILES
C(=O)(NCC)C
INCHI
InChI=1S/C4H9NO/c1-3-5-4(2)6/h3H2,1-2H3,(H,5,6)
FORMULA
C4H9NO

Common name
N-ethylacetamide
IUPAC name
N-ethylacetamide
Molecular weight
87.120
clogP
-0.038
clogS
-0.956
Frequency
0.0089
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
29.1
Number of Rings
0
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
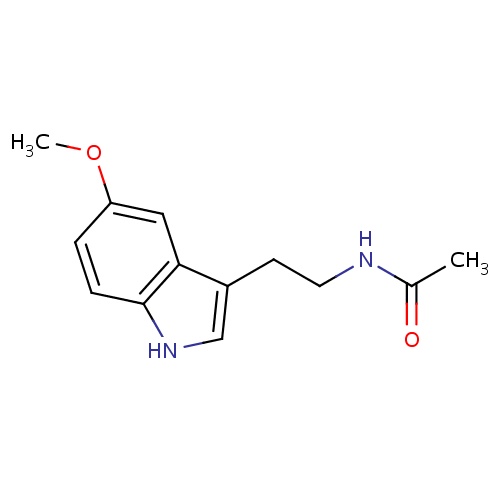
| FDBD00916 | Melatonin |
|
Antioxidants; Hypnotics and Sedatives; Central Nervous System Depressants; Nervous System; Psycholeptics; Melatonin Receptor Agonists; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; | Used orally for jet lag, insomnia, shift-work disorder, circadian rhythm disorders in the blind (evidence for efficacy), and benzodiazepine and nicotine withdrawal. Evidence indicates that melatonin is likely effective for treating circadian rhythm sleep disorders in blind children and adults. It has received FDA orphan drug status as an oral medication for this use. A number of studies have shown that melatonin may be effective for treating sleep-wake cycle disturbances in children and adolescents with mental retardation, autism, and other central nervous system disorders. It appears to decrease the time to fall asleep in children with developmental disabilities, such as cerebral palsy, autism, and mental retardation. It may also improve secondary insomnia associated with various sleep-wake cycle disturbances. Other possible uses for which there is some evidence for include: benzodiazepine withdrawal, cluster headache, delayed sleep phase syndrome (DSPS), primary insomnia, jet lag, nicotine withdrawal, preoperative anxiety and sedation, prostate cancer, solid tumors (when combined with IL-2 therapy in certain cancers), sunburn prevention (topical use), tardive dyskinesia, thrombocytopenia associated with cancer, chemotherapy and other disorders. |
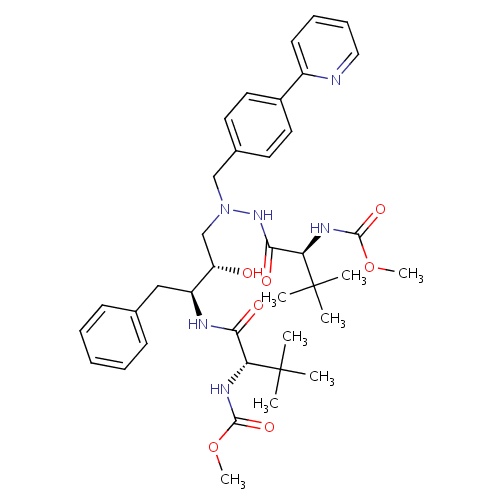
| FDBD00923 | Atazanavir |
|
Anti-HIV Agents; Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Used in combination with other antiretroviral agents for the treatment of HIV-1 infection, as well as postexposure prophylaxis of HIV infection in individuals who have had occupational or nonoccupational exposure to potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. |
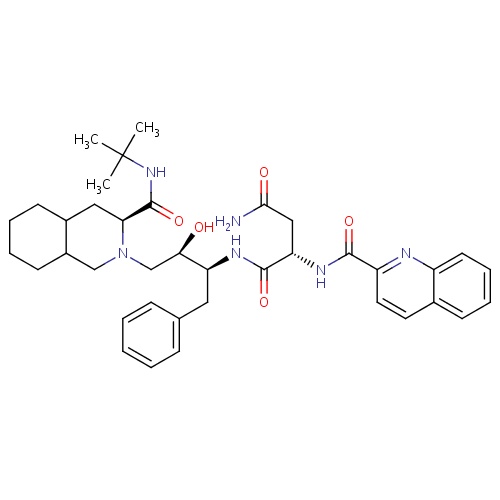
| FDBD01076 | Saquinavir |
|
Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of HIV-1 with advanced immunodeficiency together with antiretroviral nucleoside analogues. |
| FDBD01251 | Lopinavir |
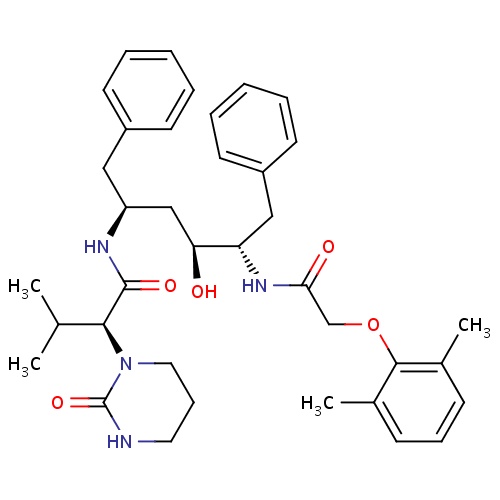
|
Anti-HIV Agents; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with other antiretroviral agents for the treatment of HIV-infection. |
| FDBD01392 | Lacosamide |

|
Anticonvulsants; Nervous System; Antiepileptics; | Lacosamide is indicated for adjunctive therapy for partial onset seizures in patients with epilepsy over 17 years old. Injection is indicated for short term use when oral therapy is not feasible. |
| FDBD01421 | Agomelatine |

|
Antidepressive Agents; Nervous System; Antidepressants; Psychoanaleptics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; | Agomelatine is indicated for the treatment of major depressive episodes in adults. |
| FDBD01424 | Peramivir |
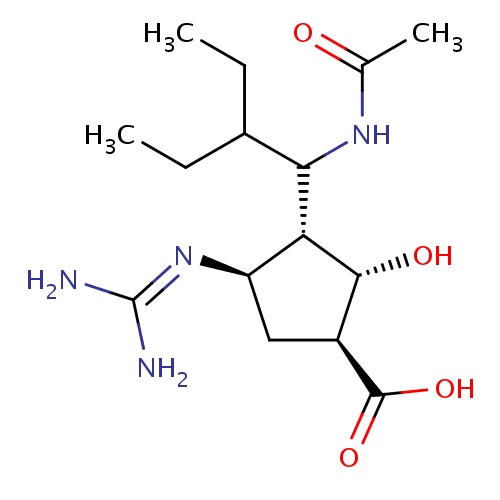
|
Antiviral Agents; Enzyme Inhibitors; | Investigated for use/treatment in influenza. |
| FDBD01539 | Tauroursodeoxycholic acid |
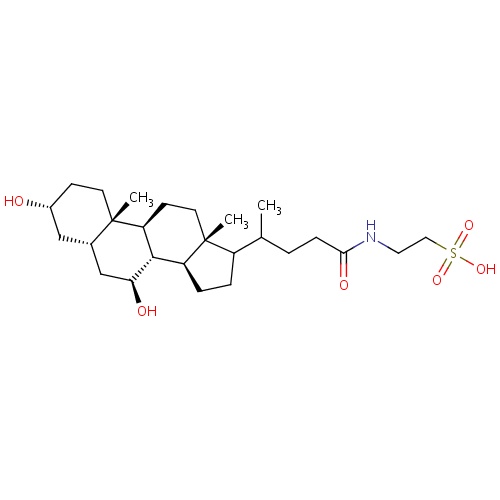
|
Antiviral Agents; Cholagogues and Choleretics; Cholesterol Absorption Inhibitors; | Used in the treatment of cholesterol gallstones. Tauroursodeoxycholic acid is also being investigated for use in several conditions such as Primary Biliary Cirrhosis (PBC), insulin resistance, amyloidosis, Cystic Fibrosis, Cholestasis, and Amyotrophic Lateral Sclerosis. |
| FDBD01640 | Eliglustat |
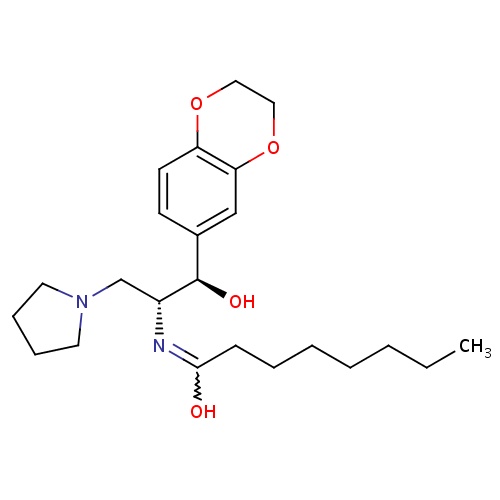
|
Enzyme Inhibitors; Alimentary Tract and Metabolism; Various Alimentary Tract and Metabolism Products; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Eliglustat is indicated for the long-term treatment of type 1 Gaucher disease in patients who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) in treatment-naive and treatment-experienced adult patients. |
| FDBD01652 | Cobicistat |
|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. |
26 ,
3
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1oth_ligand_3_16.mol2 | 1oth | 1 | -6.36 | CCNC(=O)C | 6 |
| 1csi_ligand_3_965.mol2 | 1csi | 1 | -6.17 | CCNC(=O)C | 6 |
| 1css_ligand_3_965.mol2 | 1css | 1 | -6.17 | CC(=O)NCC | 6 |
| 1kvo_ligand_3_170.mol2 | 1kvo | 1 | -6.17 | C(NC(=O)C)C | 6 |
| 1kvo_ligand_3_93.mol2 | 1kvo | 1 | -6.17 | C(NC(=O)C)C | 6 |
| 2g1r_ligand_2_9.mol2 | 2g1r | 1 | -6.17 | C(NC(=O)C)C | 6 |
944 ,
95

