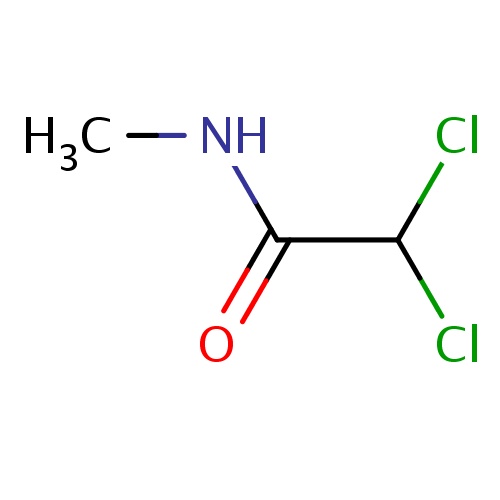
Common name
2,2-dichloro-N-methyl-acetamide
IUPAC name
2,2-dichloro-N-methyl-acetamide
SMILES
ClC(Cl)C(=O)NC
Common name
2,2-dichloro-N-methyl-acetamide
IUPAC name
2,2-dichloro-N-methyl-acetamide
SMILES
ClC(Cl)C(=O)NC
INCHI
InChI=1S/C3H5Cl2NO/c1-6-3(7)2(4)5/h2H,1H3,(H,6,7)
FORMULA
C3H5Cl2NO

Common name
2,2-dichloro-N-methyl-acetamide
IUPAC name
2,2-dichloro-N-methyl-acetamide
Molecular weight
141.984
clogP
0.853
clogS
-1.523
Frequency
0.0007
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
29.1
Number of Rings
0
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
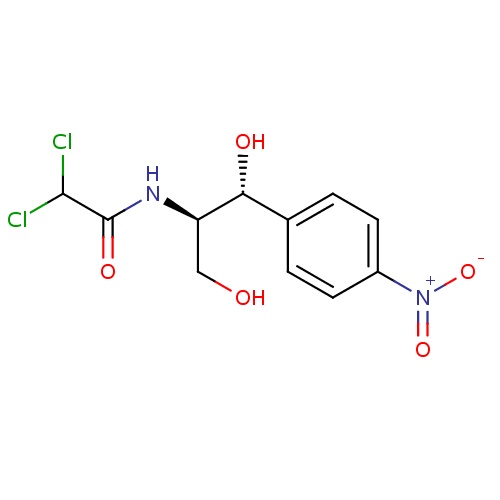
| FDBD00322 | Chloramphenicol |
|
Anti-Bacterial Agents; Protein Synthesis Inhibitors; Anti-Acne Preparations; Antibiotics; Ophthalmologicals; Sensory Organs; Genito Urinary System and Sex Hormones; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Dermatologicals; Gynecological Antiinfectives and Antiseptics; Anti-Acne Preparations for Topical Use; Antiinfectives; Antiinfectives for Treatment of Acne; Antibacterials for Intramammary Use; Otologicals; Antibiotics for Topical Use; Ophthalmological and Otological Preparations; Amphenicols; Amphenicols for Intramammary Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | Used in treatment of cholera, as it destroys the vibrios and decreases the diarrhea. It is effective against tetracycline-resistant vibrios. It is also used in eye drops or ointment to treat bacterial conjunctivitis. |
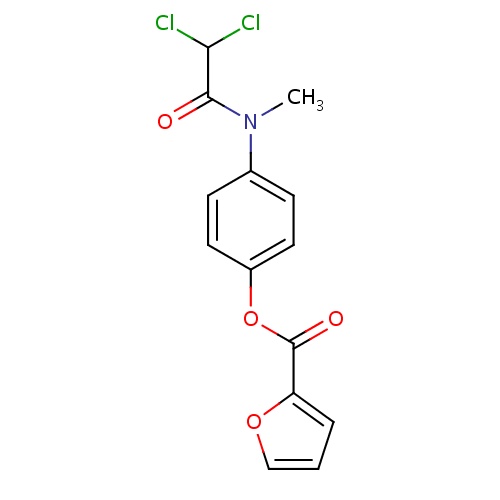
| FDBD01512 | Diloxanide |
|
Diloxanide is used alone as a primary agent in the treatment of asymptomatic (cyst passers) intestinal amebiasis caused by Entamoeba histolytica. Diloxanide may also be used concurrently, or sequentially, with other agents such as the nitroimidazoles (eg. metronidazole) in the treatment of invasive or extraintestinal forms of amebiasis. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4djo_ligand_2_104.mol2 | 4djo | 1 | -5.81 | C(Cl)(Cl)C(=O)NC | 7 |
| 4zow_ligand_2_0.mol2 | 4zow | 1 | -5.80 | C(Cl)(Cl)C(=O)NC | 7 |
| 1ct8_ligand_2_41.mol2 | 1ct8 | 1 | -5.65 | CNC(=O)C(Cl)Cl | 7 |
| 4djo_ligand_3_454.mol2 | 4djo | 0.842105 | -6.10 | C(Cl)(Cl)C(=O)NCC | 8 |
| 1ct8_ligand_3_131.mol2 | 1ct8 | 0.842105 | -5.82 | CCNC(=O)C(Cl)Cl | 8 |
| 4zow_ligand_1_0.mol2 | 4zow | 0.75 | -5.65 | C(Cl)(Cl)C(=O)N | 6 |
| 4djo_ligand_1_14.mol2 | 4djo | 0.75 | -5.61 | C(Cl)(Cl)C(=O)N | 6 |
| 1ct8_ligand_1_7.mol2 | 1ct8 | 0.75 | -5.58 | ClC(Cl)C(=O)N | 6 |
| 2gde_ligand_2_45.mol2 | 2gde | 0.722222 | -5.15 | N(C=O)CCCl | 6 |
1423 ,
143

