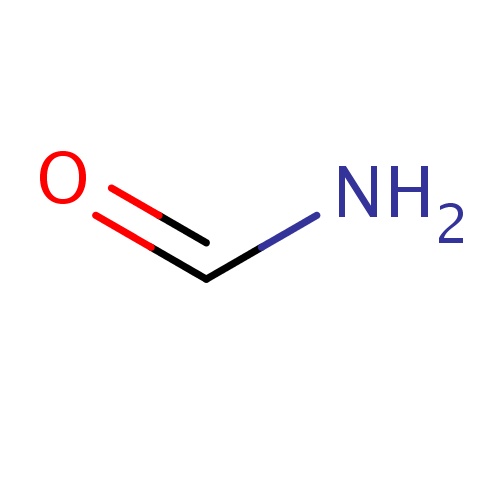
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
INCHI
InChI=1S/CH3NO/c2-1-3/h1H,(H2,2,3)
FORMULA
CH3NO

Common name
formamide
IUPAC name
formamide
Molecular weight
45.041
clogP
-0.474
clogS
0.761
Frequency
0.1240
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
43.09
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01392 | Lacosamide |

|
Anticonvulsants; Nervous System; Antiepileptics; | Lacosamide is indicated for adjunctive therapy for partial onset seizures in patients with epilepsy over 17 years old. Injection is indicated for short term use when oral therapy is not feasible. |
| FDBD01393 | Dalbavancin |

|
Anti-Bacterial Agents; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Glycopeptide Antibacterials; | Dalbavancin is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible and methicillin-resistant strains), S. pyogenes, S. agalactiae, and S. anginosus group (including S. anginosus, S. intermedius, and S. constellatus). It is administered as a 30 minute IV infusion in a two-dose regimen of 1000 mg followed by 500 mg one week later. |
| FDBD01394 | Rivaroxaban |

|
Antithrombotic Agents; Blood and Blood Forming Organs; Direct Factor Xa Inhibitors; Factor Xa Inhibitors; CYP3A4 Inhibitors; | Rivaroxaban is indicated for the prevention of venous thromboembolic events (VTE) in patients who have undergone total hips replacements and total knee replacement surgery; prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE); to reduce risk of recurrent DVT and/or PE. Due to a lack of safety studies, it is not recommended for use in those under 18 years old. Its use is also not recommended in those with severe renal impairment (. |
| FDBD01395 | Avanafil |

|
Genito Urinary System and Sex Hormones; Drugs Used in Erectile Dysfunction; Urological Agents; CYP3A4 Inhibitors; | Treatment of erectile dysfunction in males. |
| FDBD01402 | Alvimopan |

|
Gastrointestinal Agents; Alimentary Tract and Metabolism; Drugs for Constipation; Peripheral Opioid Receptor Antagonists; | Used to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection surgery with primary anastomosis. Also investigated for use in the treatment of pain (acute or chronic). |
| FDBD01405 | Amisulpride |
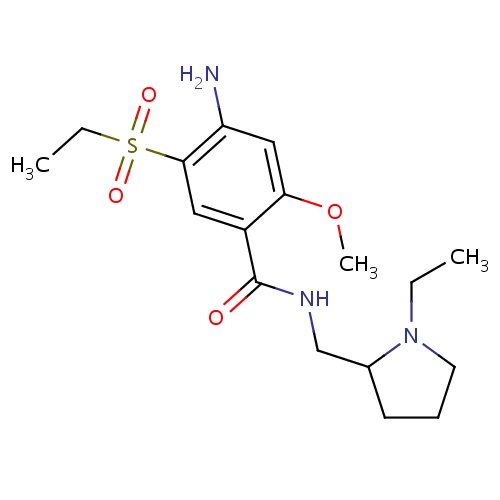
|
Antipsychotic Agents; Dopamine Antagonists; Nervous System; Psycholeptics; Benzamides; | Investigated for use/treatment in schizophrenia and schizoaffective disorders, mania in bipolar disorder, and depression. |
| FDBD01406 | Simeprevir |

|
Simeprevir is indicated in patient's with hepatitis C virus (HCV) genotype 1 for the treatment of chronic hepatitis as a combination therapy, which includes peginterferon alfa and ribavirin. | |
| FDBD01410 | Telavancin |

|
Anti-Bacterial Agents; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Glycopeptide Antibacterials; | Treatment of complicated skin infections caused by gram-positive bacteria like methicillin-susceptible or -resistant Staphylococcus aureus, vancomycin-susceptible Enterococcus faecalis, and Streptococcus pyogenes, Streptococcus agalactiae, or Streptococcus anginosus group. |
| FDBD01414 | Armodafinil |

|
Central Nervous System Stimulants; Neuroprotective Agents; Nervous System; Psychoanaleptics; Centrally Acting Sympathomimetics; Psychostimulants, Agents Used for Adhd and Nootropics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; Wakefulness-Promoting Agents; | Investigated for use/treatment in sleep disorders, obstructive sleep apnea, schizophrenia and schizoaffective disorders, depression, and bipolar disorders. |
| FDBD01418 | Prucalopride |
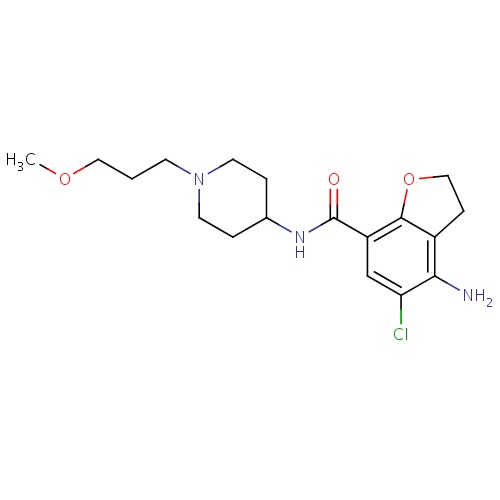
|
Alimentary Tract and Metabolism; Drugs for Constipation; | Investigated for use/treatment in constipation, ileus, and pediatric indications. |
361 ,
37
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vl1_ligand_frag_1.mol2 | 2vl1 | 1 | -5.59 | C(=O)N | 3 |
| 1wdn_ligand_frag_3.mol2 | 1wdn | 1 | -5.56 | C(=O)N | 3 |
| 1db5_ligand_frag_4.mol2 | 1db5 | 1 | -5.51 | C(=O)N | 3 |
| 2ntf_ligand_frag_10.mol2 | 2ntf | 1 | -5.51 | C(=O)N | 3 |
| 4pml_ligand_frag_1.mol2 | 4pml | 1 | -5.51 | C(=O)N | 3 |
| 4xmb_ligand_frag_8.mol2 | 4xmb | 1 | -5.51 | C(=O)N | 3 |
| 4tjy_ligand_frag_1.mol2 | 4tjy | 1 | -5.50 | C(=O)N | 3 |
| 4f1q_ligand_frag_4.mol2 | 4f1q | 1 | -5.49 | C(=O)N | 3 |
4319 ,
432

