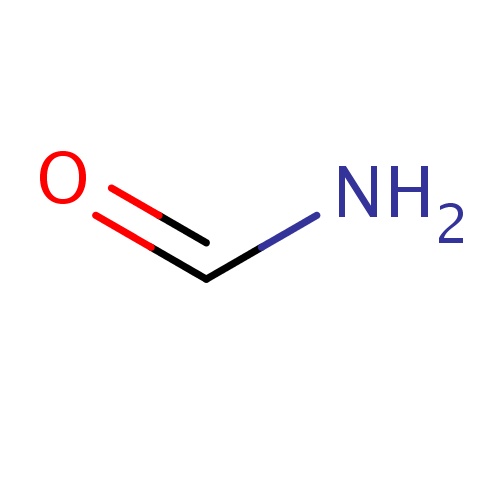
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
INCHI
InChI=1S/CH3NO/c2-1-3/h1H,(H2,2,3)
FORMULA
CH3NO

Common name
formamide
IUPAC name
formamide
Molecular weight
45.041
clogP
-0.474
clogS
0.761
Frequency
0.1240
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
43.09
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01532 | Ivacaftor |
|
Respiratory System; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of cystic fibrosis (CF) in patients age 6 years and older who have a G551D mutation in the CFTR gene. |
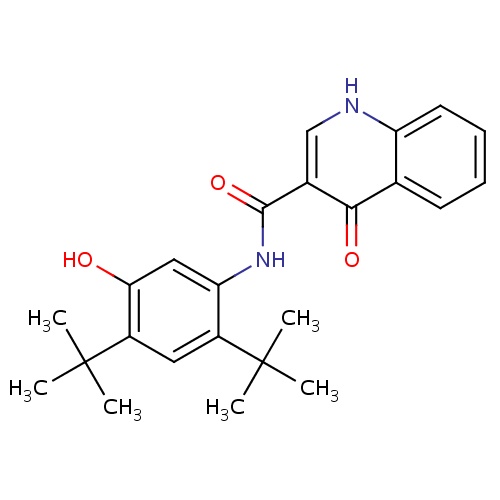
| FDBD01537 | Lomitapide |
|
Hypolipidemic Agents; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; CYP3A4 Inhibitors; | Used in homozygous familial hypercholesterolemia (HoFH) patients to reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein B (apo B), and non-high-density lipoprotein cholesterol (non-HDL-C). |
| FDBD01538 | Vismodegib |

|
Antineoplastic Agents; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | Vismodegib is used for treating locally advanced or metastatic basal cell carcinoma in patients whose carcinoma has recurred after surgery, and in patients who are not candidates for surgery or radiation. |
| FDBD01539 | Tauroursodeoxycholic acid |
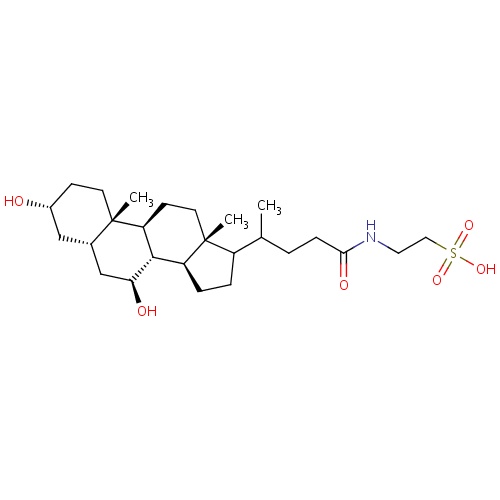
|
Antiviral Agents; Cholagogues and Choleretics; Cholesterol Absorption Inhibitors; | Used in the treatment of cholesterol gallstones. Tauroursodeoxycholic acid is also being investigated for use in several conditions such as Primary Biliary Cirrhosis (PBC), insulin resistance, amyloidosis, Cystic Fibrosis, Cholestasis, and Amyotrophic Lateral Sclerosis. |
| FDBD01540 | Spaglumic Acid |
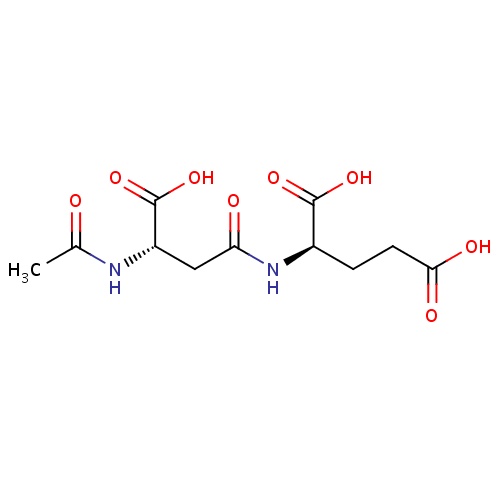
|
Mast Cell Stabilizers; Respiratory System; Ophthalmologicals; Sensory Organs; Nasal Preparations; Decongestants and Antiallergics; Antiallergic Agents, Excl. Corticosteroids; | Used in patients with allergic conjunctivitis. |
| FDBD01550 | Boceprevir |
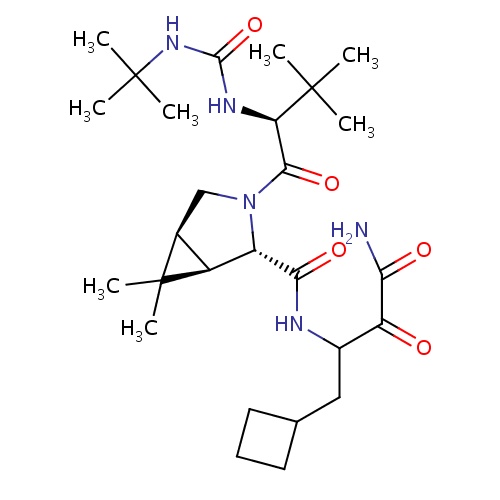
|
Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Treatment of chronic hepatitis C genotype 1 in patients that have a compensated liver (as a result of liver diseases like cirrhosis) and are previously untreated or therapy with peginterferon alfa and ribavirin has failed. |
| FDBD01557 | Carfilzomib |

|
Antineoplastic Agents; Antineoplastic and Immunomodulating Agents; | Carfilzomib is indicated for the treatment of patients with multiple myeloma who have received at least two prior therapies including bortezomib and an immunomodulatory agent and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate. |
| FDBD01558 | Linaclotide |
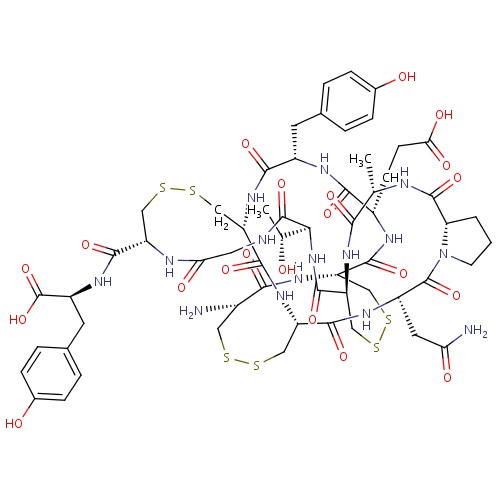
|
Alimentary Tract and Metabolism; Drugs for Constipation; | Treatment of irritable bowel syndrome (IBS) with constipation and chronic idiopathic constipation. |
| FDBD01559 | Mirabegron |
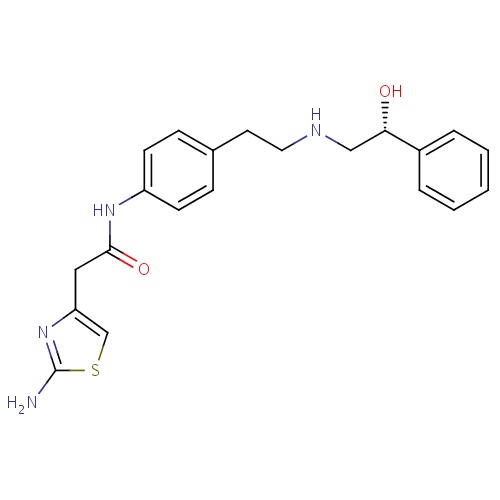
|
Muscle Relaxants, Genitourinary; Genito Urinary System and Sex Hormones; Drugs for Urinary Frequency and Incontinence; Urological Agents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Adrenergic beta-3 Receptor Agonists; | Mirabegron is a beta-3 adrenergic agonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. |
| FDBD01564 | Ponatinib |
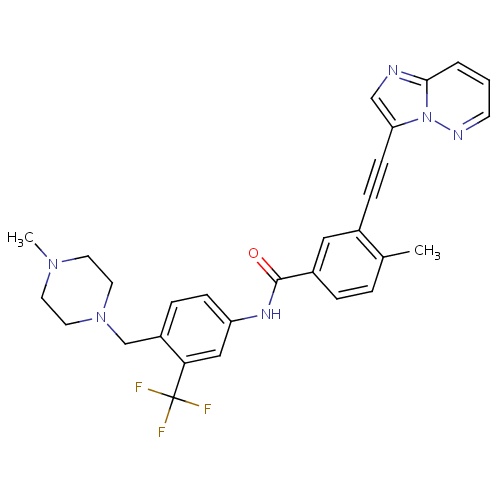
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; BSEP/ABCB11 Inhibitors; | Ponatinib is indicated for the treatment of adult patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) that is resistant or intolerant to prior tyrosine kinase inhibitor therapy or Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL) that is resistant or intolerant to prior tyrosine kinase inhibitor therapy. |
361 ,
37
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vl1_ligand_frag_1.mol2 | 2vl1 | 1 | -5.59 | C(=O)N | 3 |
| 1wdn_ligand_frag_3.mol2 | 1wdn | 1 | -5.56 | C(=O)N | 3 |
| 1db5_ligand_frag_4.mol2 | 1db5 | 1 | -5.51 | C(=O)N | 3 |
| 2ntf_ligand_frag_10.mol2 | 2ntf | 1 | -5.51 | C(=O)N | 3 |
| 4pml_ligand_frag_1.mol2 | 4pml | 1 | -5.51 | C(=O)N | 3 |
| 4xmb_ligand_frag_8.mol2 | 4xmb | 1 | -5.51 | C(=O)N | 3 |
| 4tjy_ligand_frag_1.mol2 | 4tjy | 1 | -5.50 | C(=O)N | 3 |
| 4f1q_ligand_frag_4.mol2 | 4f1q | 1 | -5.49 | C(=O)N | 3 |
4319 ,
432

