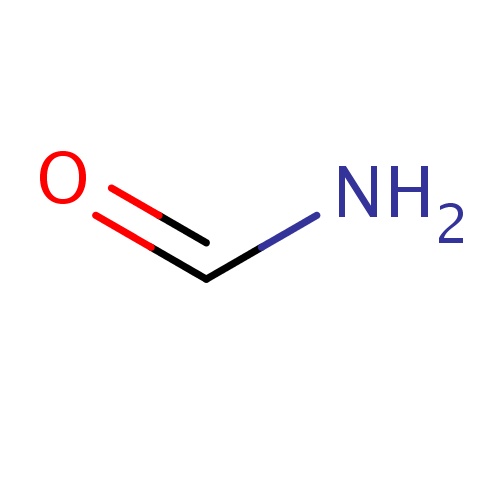
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
INCHI
InChI=1S/CH3NO/c2-1-3/h1H,(H2,2,3)
FORMULA
CH3NO

Common name
formamide
IUPAC name
formamide
Molecular weight
45.041
clogP
-0.474
clogS
0.761
Frequency
0.1240
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
43.09
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01423 | Apixaban |

|
Antithrombotic Agents; Blood and Blood Forming Organs; Direct Factor Xa Inhibitors; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Factor Xa Inhibitors; CYP3A4 Inhibitors; | Apixaban is to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. It has also been used to lower the risk of developing venous thrombosis post-orthopedic surgical procedures. |
| FDBD01430 | Vilazodone |

|
Serotonin Uptake Inhibitors; Serotonin 5-HT1 Receptor Agonists; Serotonin Receptor Agonists; Antidepressive Agents; Nervous System; Antidepressants; Psychoanaleptics; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Vilazodone is approved for treatment of acute episodes of major depression. Labeling of vilazodone describes an increased risk of suicidal thoughts in children, adolescents and young adults. The use of vilazodone in pediatrics is not indicated. Its use with monoamine oxidase inhibitors (MAOI) is contraindicated due to increased risk of serotonin syndome. Once the MAOI is discontinued, a 14-day washout period must pass before starting vilazodone. |
| FDBD01435 | Dabigatran etexilate |
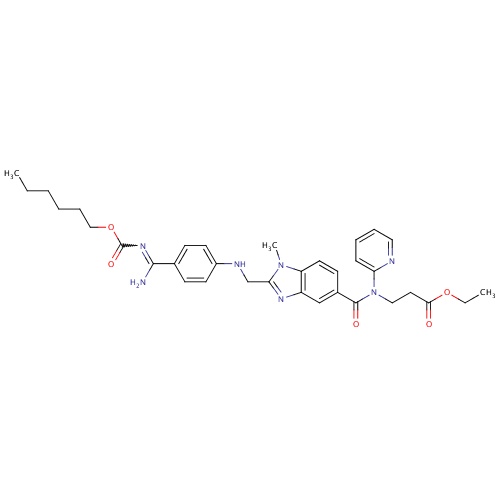
|
Antithrombins; Direct Thrombin Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; | Dabigatran is indicated for the prevention of venous thromboembolic events in patients who have undergone elective hip or knee replacement surgery (based on RE-NOVATE, RE-MODEL, and RE-MOBILIZE trials). In 2010, it was approved in the US and Canada for prevention of stroke and systemic embolism in patients with atrial fibrillation (approval based on the RE-LY trial). Contraindications: severe renal impairment (CrCL . |
| FDBD01436 | Arbekacin |

|
Anti-Infective Agents; Aminoglycosides; Antibiotics; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Aminoglycoside Antibacterials; | Arbekacin is used for the short term treatment of multi-resistant bacterial infections, such as methicillin-resistant . |
| FDBD01439 | Degarelix |

|
Gonadotropin-releasing hormone antagonist; Antineoplastic and Immunomodulating Agents; Endocrine Therapy; Hormone Antagonists and Related Agents; | Degaralix is used for the management of advanced prostate cancer. |
| FDBD01475 | Capsaicin |
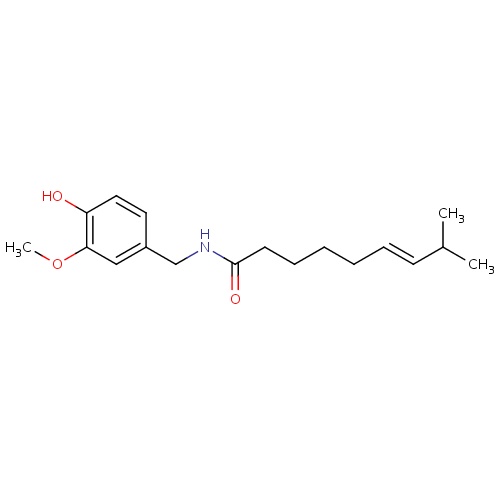
|
Anesthetics, Local; Antipruritics; Anesthetics; Sensory System Agents; Musculo-Skeletal System; Nervous System; Topical Products for Joint and Muscular Pain; Capsaicin and Similar Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | The capsaicin 8% patch is indicated in the treatment of neuropathic pain associated with post-herpetic neuralgia. There are multiple topical capsaicin formulations available, including creams and solutions, indicated for temporary analgesia in muscle and join pain as well as neuropathic pain. |
| FDBD01481 | Ganirelix |
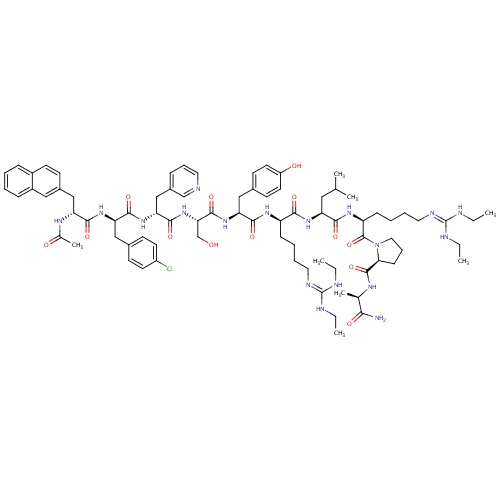
|
Hormone Antagonists; Pituitary and Hypothalamic Hormones and Analogues; Systemic Hormonal Preparations, Excl. Sex Hormones and Insulins; Anti-Gonadotropin-Releasing Hormones; Hypothalamic Hormones; | For the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation. |
| FDBD01484 | Histrelin |
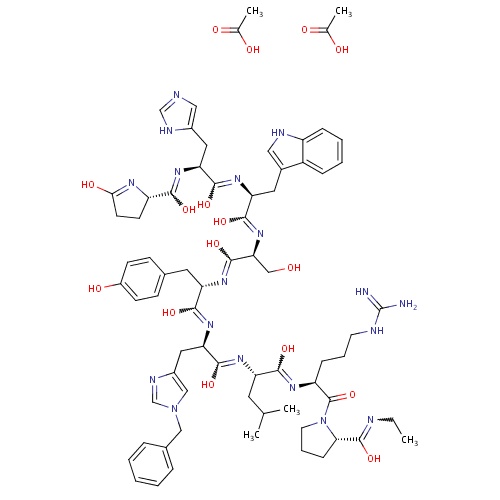
|
Histrelin acetate has two indications | |
| FDBD01486 | Lanreotide |
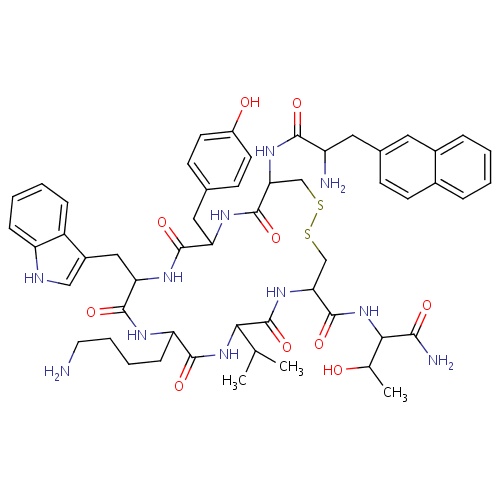
|
Antineoplastic Agents; Pituitary and Hypothalamic Hormones and Analogues; Systemic Hormonal Preparations, Excl. Sex Hormones and Insulins; Hypothalamic Hormones; Somatostatin and Analogues; | Lanreotide is a somatostatin analog approved for treatment of neuroendocrine tumours and acromegaly. (2). |
| FDBD01494 | Nepafenac |
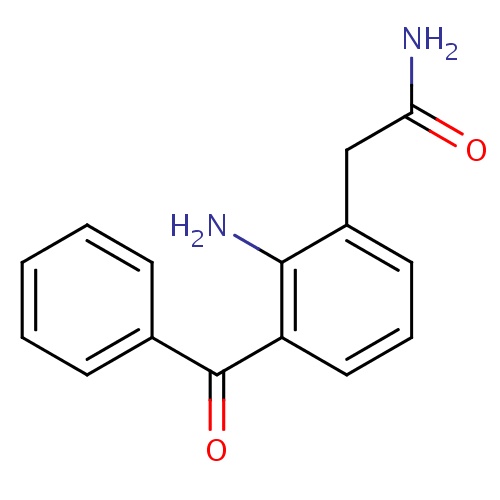
|
Anti-Inflammatory Agents; Anti-Inflammatory Agents, Non-Steroidal; Prodrugs; Ophthalmologicals; Sensory Organs; | For the treatment of pain and inflammation associated with cataract surgery. |
361 ,
37
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vl1_ligand_frag_1.mol2 | 2vl1 | 1 | -5.59 | C(=O)N | 3 |
| 1wdn_ligand_frag_3.mol2 | 1wdn | 1 | -5.56 | C(=O)N | 3 |
| 1db5_ligand_frag_4.mol2 | 1db5 | 1 | -5.51 | C(=O)N | 3 |
| 2ntf_ligand_frag_10.mol2 | 2ntf | 1 | -5.51 | C(=O)N | 3 |
| 4pml_ligand_frag_1.mol2 | 4pml | 1 | -5.51 | C(=O)N | 3 |
| 4xmb_ligand_frag_8.mol2 | 4xmb | 1 | -5.51 | C(=O)N | 3 |
| 4tjy_ligand_frag_1.mol2 | 4tjy | 1 | -5.50 | C(=O)N | 3 |
| 4f1q_ligand_frag_4.mol2 | 4f1q | 1 | -5.49 | C(=O)N | 3 |
4319 ,
432

