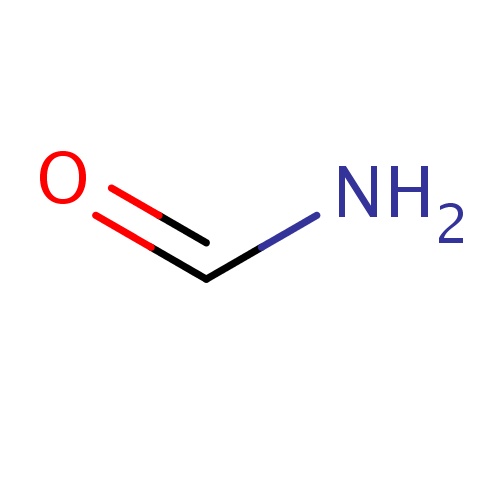
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
Common name
formamide
IUPAC name
formamide
SMILES
C(=O)N
INCHI
InChI=1S/CH3NO/c2-1-3/h1H,(H2,2,3)
FORMULA
CH3NO

Common name
formamide
IUPAC name
formamide
Molecular weight
45.041
clogP
-0.474
clogS
0.761
Frequency
0.1240
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
43.09
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01633 | Aliskiren |
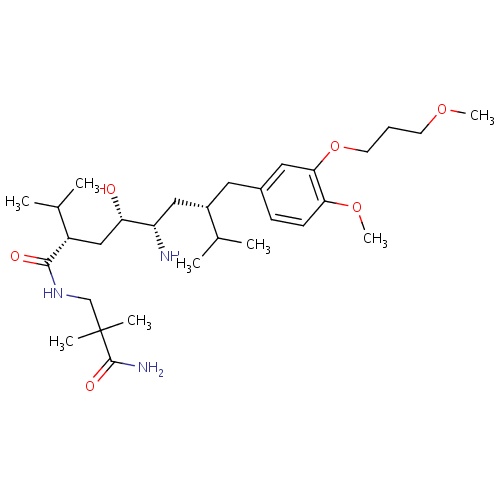
|
Cardiovascular System; Agents Acting on the Renin-Angiotensin System; Renin-Inhibitors; CYP3A4 Inhibitors; | For the treatment of hypertension, to lower blood pressure. |
| FDBD01634 | Ledipasvir |

|
Antiviral Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; | Ledipasvir in combination with sofosbuvir, or in combination with sofosbuvir and ribavirin, is indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection in adults. |
| FDBD01640 | Eliglustat |
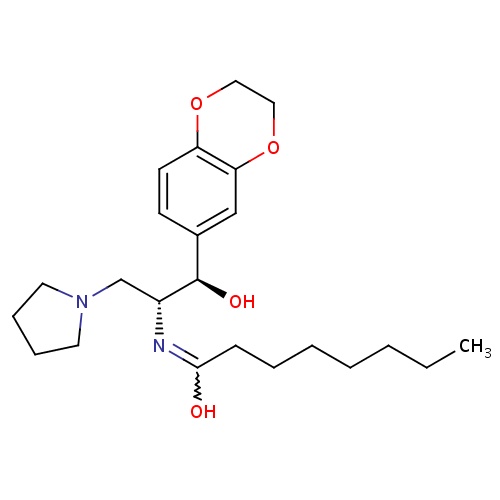
|
Enzyme Inhibitors; Alimentary Tract and Metabolism; Various Alimentary Tract and Metabolism Products; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Eliglustat is indicated for the long-term treatment of type 1 Gaucher disease in patients who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) in treatment-naive and treatment-experienced adult patients. |
| FDBD01650 | Avibactam |

|
AVYCAZ (ceftazidime-avibactam), in combination with metronidazole, is indicated for the treatment of complicated intra-abdominal infections caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Providencia stuartii, Enterobacter cloacae, Klebsiella oxytoca, and Pseudomonas aeruginosa in patients 18 years or older. | |
| FDBD01652 | Cobicistat |
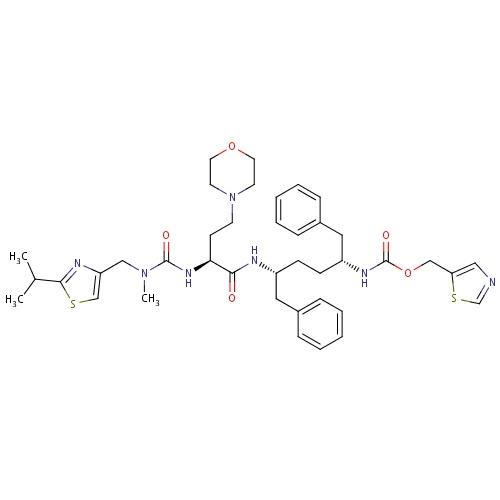
|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. |
| FDBD01656 | Tasimelteon |

|
Hypnotics and Sedatives; Nervous System; Psycholeptics; Melatonin Receptor Agonists; | Tasimelteon is indicated for the treatment of Non-24-Hour Sleep-Wake Disorder (N24HSWD). |
| FDBD01659 | Edoxaban |
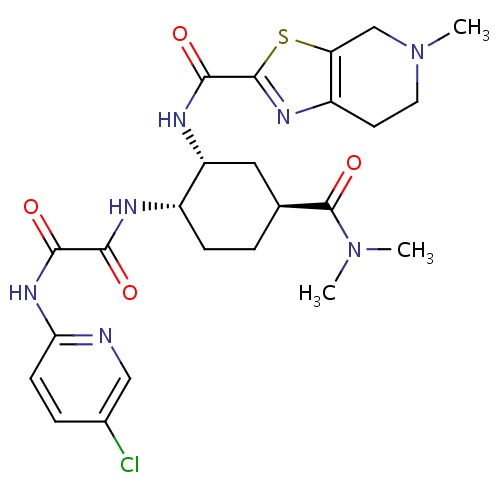
|
Factor Xa Inhibitors; | Edoxaban is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). However, it should not be used in patients with creatinine clearance (CrCL) > 95 mL/min because of increased risk of ischemic stroke compared to warfarin at the highest dose studied (60 mg). It is also indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5-10 days of initial therapy with a parenteral anticoagulant. |
| FDBD01661 | Lenvatinib |

|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; CYP3A4 Inhibitors; | Lenvatinib is indicated for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer. |
| FDBD01680 | Somatostatin |
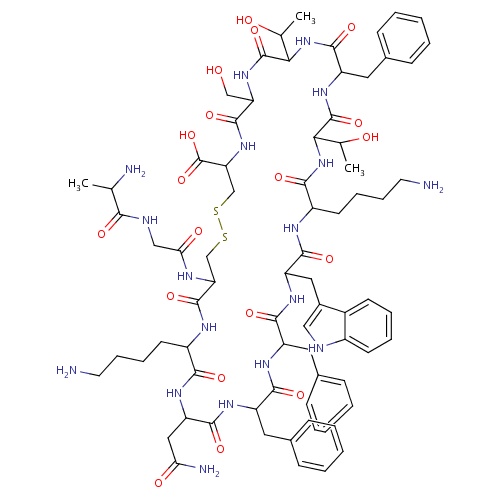
|
Hormones; Pituitary and Hypothalamic Hormones and Analogues; Systemic Hormonal Preparations, Excl. Sex Hormones and Insulins; Hypothalamic Hormones; Somatostatin and Analogues; | For the symptomatic treatment of acute bleeding from esophageal varices. Other treatment options for long-term management of the condition may be considered if necessary, once initial control has been established. |
| FDBD01682 | Daclatasvir |

|
Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Daklinza is used to treat patients who have chronic hepatitis C virus (HCV) genotype 3 infection. Daklinza is typically taken in conjunction with sofosbuvir. (2). |
361 ,
37
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vl1_ligand_frag_1.mol2 | 2vl1 | 1 | -5.59 | C(=O)N | 3 |
| 1wdn_ligand_frag_3.mol2 | 1wdn | 1 | -5.56 | C(=O)N | 3 |
| 1db5_ligand_frag_4.mol2 | 1db5 | 1 | -5.51 | C(=O)N | 3 |
| 2ntf_ligand_frag_10.mol2 | 2ntf | 1 | -5.51 | C(=O)N | 3 |
| 4pml_ligand_frag_1.mol2 | 4pml | 1 | -5.51 | C(=O)N | 3 |
| 4xmb_ligand_frag_8.mol2 | 4xmb | 1 | -5.51 | C(=O)N | 3 |
| 4tjy_ligand_frag_1.mol2 | 4tjy | 1 | -5.50 | C(=O)N | 3 |
| 4f1q_ligand_frag_4.mol2 | 4f1q | 1 | -5.49 | C(=O)N | 3 |
4319 ,
432

